Don’t Keep a Risk Register in Excel (Use Jira)
If you’re considering using Excel for your risk register, this video explains why that might not…

Jira Risk Management Workflow Tutorial
In this article, we will look at the 4 steps needed to build a Risk Register…

Using Jira for Risk Management: 8 Best Practices For 2024
When it comes to managing risks in project management, using Jira for risk management can significantly…
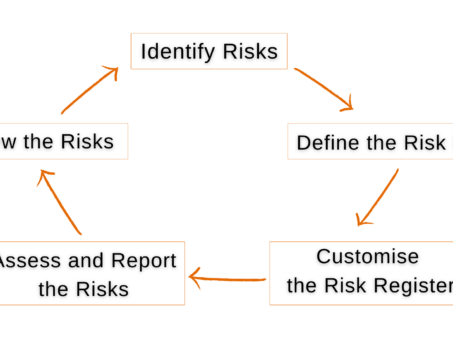
5 Steps To Tracking & Mitigating Project Risks in Jira
Project risk management is crucial for project success, especially important to keep critical risks under check…

Supporting Regulated Industries on Atlassian
On May 2, 2024 SoftComply hosted the 2nd edition of Regulated Industries workshop during Atlassian Team…

What are the FDA 21 CFR 11 Compliant Electronic Records?
Title 21 of the Code of Federal Regulations, Part 11, also known as 21 CFR 11,…

Don’t Leave Your Quality Team Behind!
Your Development team might find the instructions from Quality and Compliance team time-consuming – slowing down…

End App Fatigue with one Risk Management Solution
For decades organisations have been struggling with working in silos with different departments working with several…

HOW TO USE THE AUTOMATION ASSISTANT OF THE RISK MANAGER PLUS?
We have just released a new Automation Assistant for the SoftComply Risk Manager Plus app –…

How to Manage Information Security Risks in Jira in Compliance with ISO/IEC 27001
What is Information Security Risk Management? Information security risk management is a systematic process that involves…

Changes of Confluence Cloud in 2023: Detected by SoftComply
Confluence Cloud is constantly changing and not all the changes that are implemented by Atlassian are…

Increase your Efficiency in Confluence
Introducing the Toolbox App that Solves Three Critical Problems for Confluence Cloud Users Are you a…