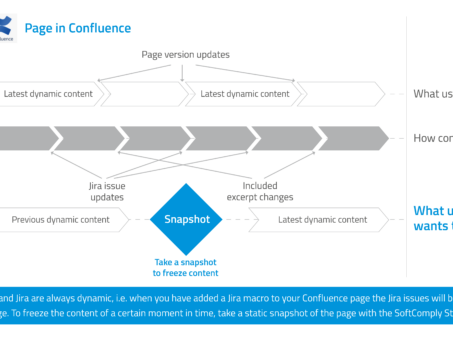
How to keep your data in Confluence static?
If you have worked with Jira and Confluence, am sure you have enjoyed the integration between…

Compliance of myBioma
The Regulatory Compliance Journey of myBioma Biome Diagnostics GmbH is an Austrian medtech start-up utilizing the…
Medical Device Compliance & Atlassian Cloud
On the 16th of October 2020, Atlassian announced radical changes to their offering. From February 2nd…