If you have worked with Jira and Confluence, am sure you have enjoyed the integration between the two. The way you can easily pull data from Jira to Confluence by creating macros on your Confluence page with all the data from Jira that you want to display is absolutely wonderful.
Now, every time you refresh the Confluence page, the content of the macro with the Jira issues will be updated reflecting the changes that have been made in Jira. This is a fantastic and seamless way of always being on top of any changes that may have occurred.
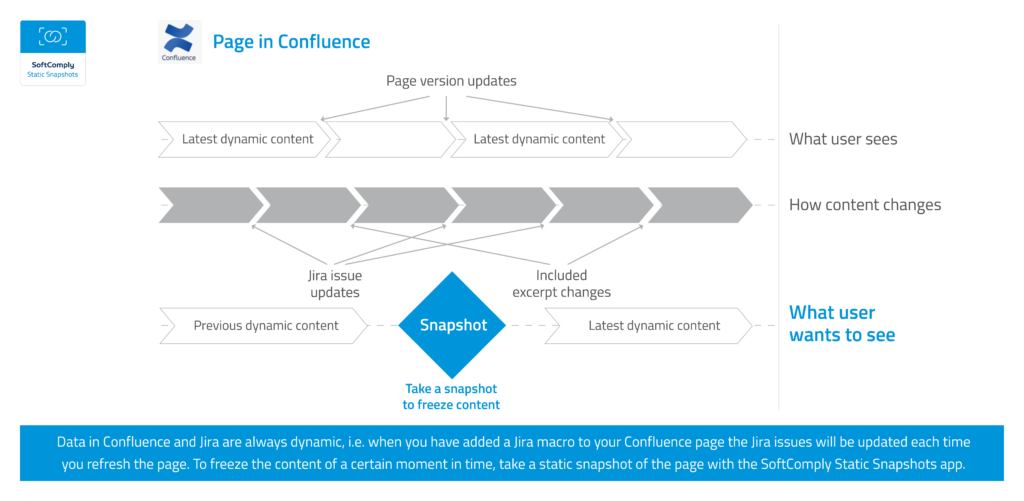
But what if you want to keep a report or snapshot of data from a certain point in time to be able to monitor progress or highlight changes afterwards? Say a meeting minutes that has a Confluence macro in it with content from Jira that you would like to store without the Jira issues being updated when you revisit the notes a few days later?
What about Confluence pages that need to be reviewed and approved by your colleagues? At the moment they may be approving a page the content of which is constantly changing. To make sure that the content of the page you reviewed and approved will not change, you should first freeze the data on the Confluence page.
SoftComply, as an Atlassian vendor for the regulated industries where constantly changing data is often an obstacle for compliance, offers an app to solve this issue.
Try the SoftComply Static Snapshots app to capture and freeze your data in Confluence!
SoftComply Static Snapshots app was developed to allow changing the dynamic data on a Confluence page to static. You can store a static snapshot of a Confluence page and you can add that snapshot to any other page that you want.
A short explanatory animation clip about the SoftComply Static Snapshots app:
You can also try out the app for free from Atlassian Marketplace.
If you have any questions or would like to see a demo, please contact us at SoftComply.