
Post-Market Surveillance & Risk Management
All product development teams let out a sigh of relief when a product is launched. The hard technical work is done, tight deadlines met (more or less), submissions completed and approved. Time to hand over to the sales and marketing group and take a well deserved break.
But other parts of the company, even in the development group, may not feel the same. Quality team starts looking more into what happens in production, new features that may be on the horizon, and the Regulatory team gets ready to hold the line when the complaints start coming in (and they will come in, not matter how good your device is).
Post-market surveillance is the process of monitoring the safety and effectiveness of a product on the market, to identify potential issue and take appropriate actions, if any is required. This process has historically been quite “reactive”, meaning that a Company monitors signals such as complaints, events that indicate potential issues AFTER something happens.
With the recent focus on Cybersecurity, post-market activities have become way more “proactive”; vulnerability monitoring allows a company to patch software BEFORE a vulnerability can be exploited.
Regardless of the device, the type of “signal” being monitored, hardware, software, etc., there is one common point (more than one actually): Risk Management.
The Risk Management File (RMF) of a medical device must be kept up to date for the whole life of a product, and beyond. The two most important post-market inputs to the RMF are without doubt Complaints and Changes.
Below is a summary of the standard post-market surveillance activities related to risk management:
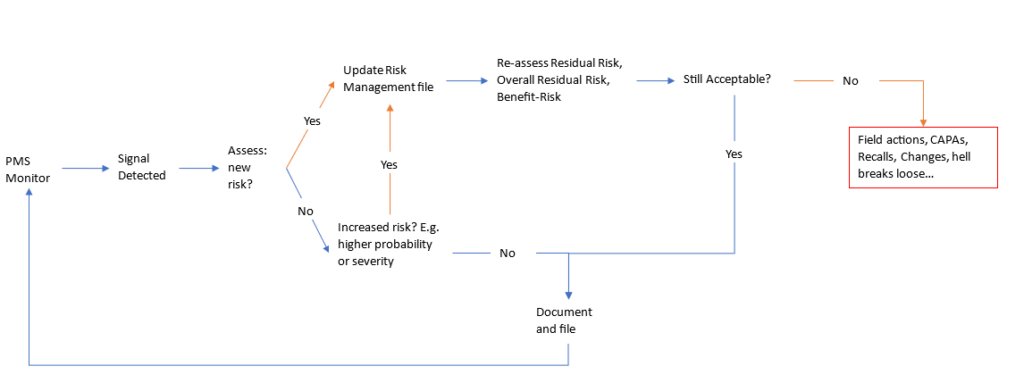
The post-market surveillance process is usually slow as complaints must be carefully examined. One of the most common observations from auditors and inspectors is that a Company took too long to act after receiving a Complaints.
Having a Risk Management system that can easily and quickly be updated can save a lot of this dead time, and automatic traceability can immediately identify potentially affected areas of the product or the system.
👉 Try out the SoftComply Risk Manager Plus to better manage your risks in Jira or book a demo to learn more.
👋 Will you attend Medica? Book a slot to meet our team there:

Recommended articles



