What is a Benefit-Risk Analysis & How To Do It?

The Benefit-Risk analysis (a.k.a. Benefit-Risk determination or Benefit-Risk ratio) is one of the most misinterpreted areas of the Risk Management process, in particular when coupled with the requirements of MDR / IVDR. And consequently one of the preferred digging points for the Notified Bodies. The spirit of the regulations and standards is “the benefit provided […]
4 Reasons Why you should Bring your Risk Register from Excel to Jira

4 Benefits of Having your Risk Register in Jira rather than Excel
SoftComply Risk Manager vs Risk Manager Plus on Jira Cloud

Comparison between the risk management apps on Atlassian Jira – the SoftComply Risk Manager and the Risk Manager Plus on Jira Cloud
In Search of a Compliance Solution that Lets You Sleep at Night

How to find the best regulatory compliance solution for medtech on Atlassian
SOFTCOMPLY RISK MANAGER IS NOW CLOUD FORTIFIED

Introduction Last week we reached an important milestone – the SoftComply Risk Manager on Jira Cloud was awarded the Cloud Fortified badge by Atlassian. The SoftComply Risk Manager is the only product risk management app on Jira and the most customisable one with Risk Management guidance, multiple views to work in and compliant Risk Reporting. To learn more about the SoftComply Risk Manager on Jira Cloud, please book your […]
How to Create a Risk Traceability Matrix in Jira
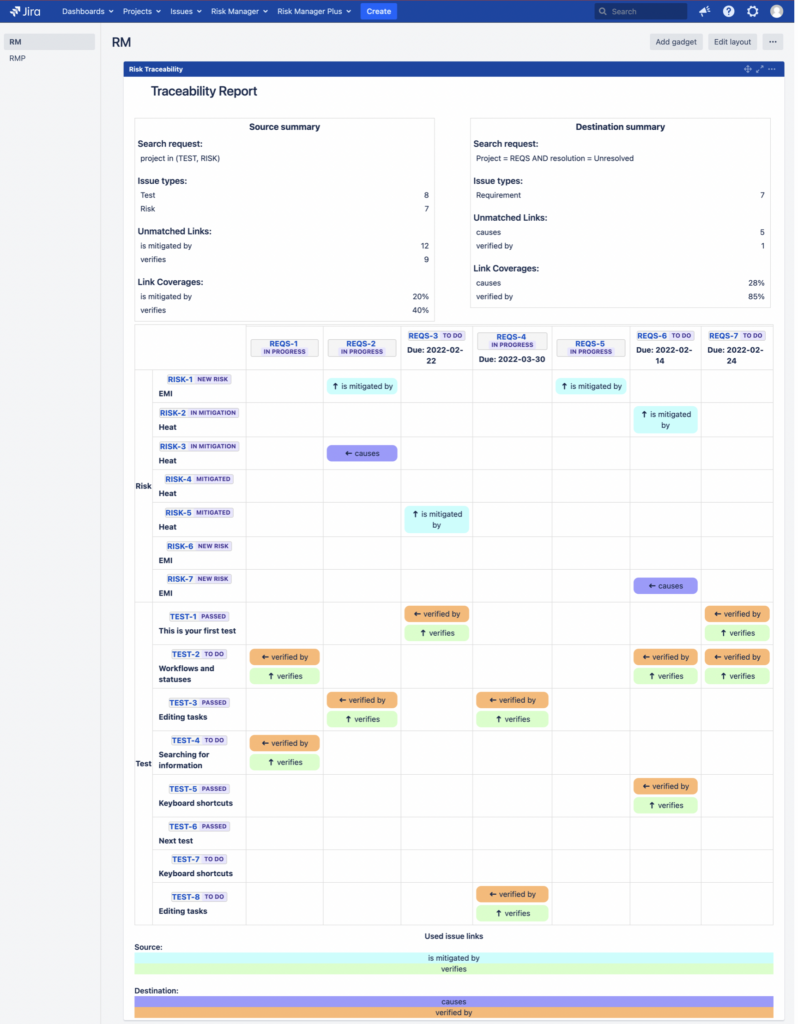
Introduction Risk Traceability is a great way to get a quick overview of the completeness of risk management process and it is often used in the regulated industries. The traceability matrix shows the coverage of risks by mitigation and verification actions. For example, in the medical device industry, both the FDA and ISO 14971 require bi-directional […]
How to Create a Risk from a Jira Support Ticket?
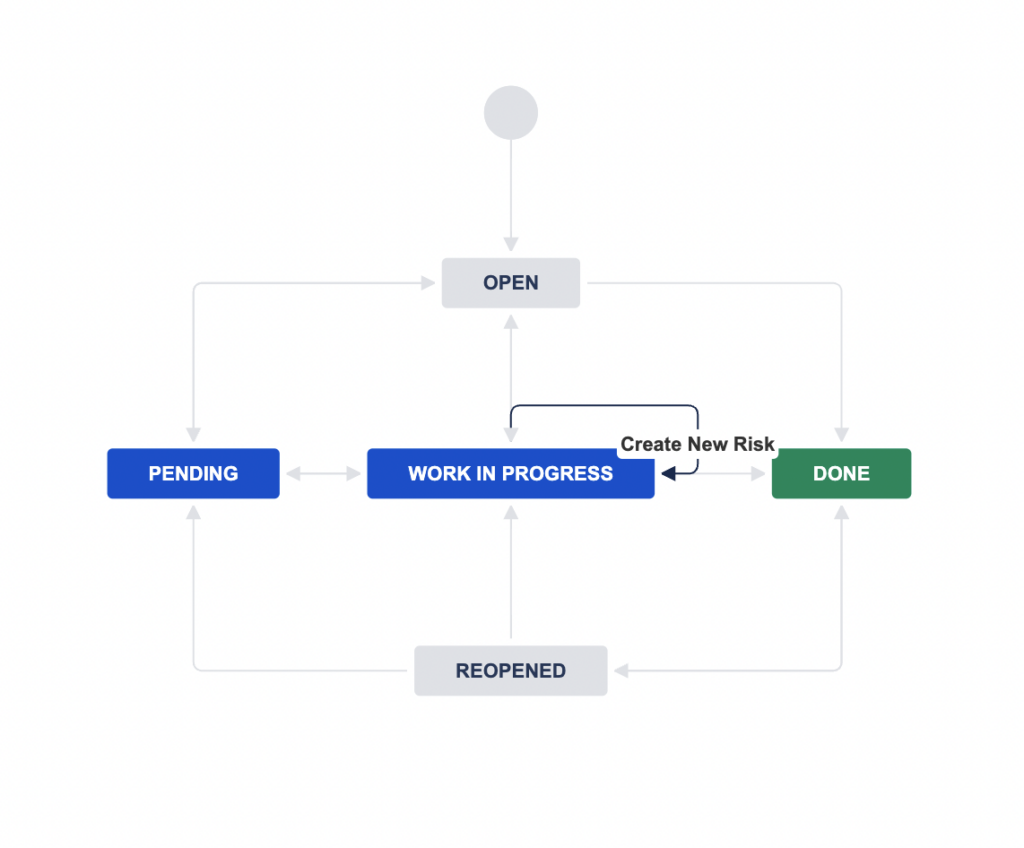
Introduction Jira is the most flexible task management tool there is. No wonder that more than 200’000 companies across the world have found it useful. It helps software teams build their products, prioritise their backlog and manage any upcoming issues within their team. Most of these companies are using Jira Service Management to collect customer […]
Surviving Risk Management Audit with Excel: a True Account on Why we Built our own Risk Management Apps on Jira

“Ok, let’s follow a few of these risk mitigation actions down to outputs and verification activities” says the auditor. A typical question. Anybody who’s ever been audited for risk management process, has been here dozens of times. While I try to bring the attention of the auditor to another topic, I know a few meters away […]
How to Report Risks in Jira
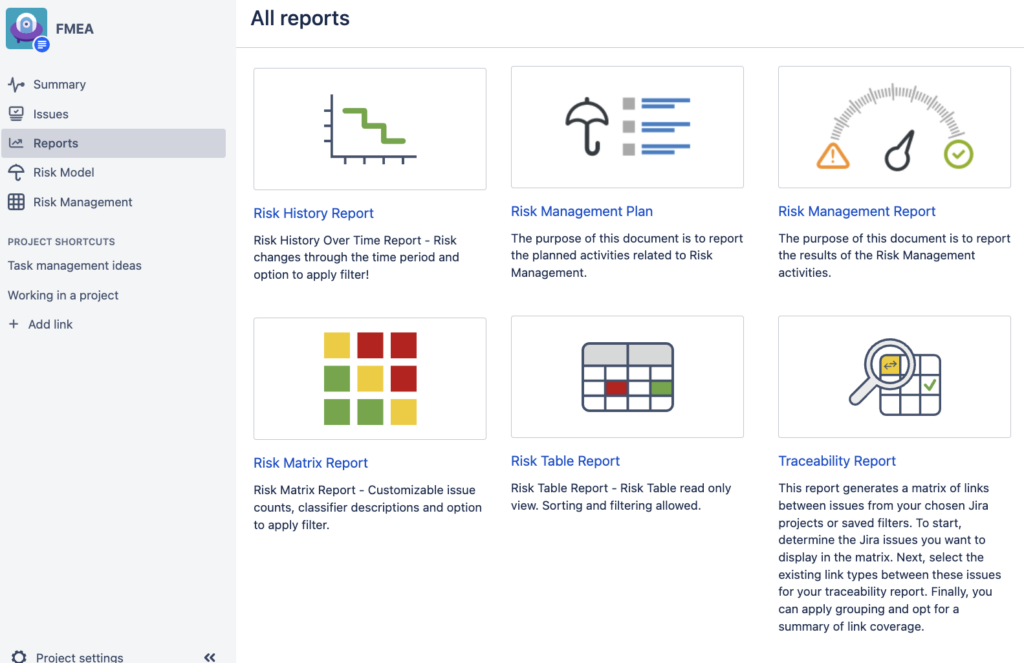
Introduction When you manage your product risks in a highly regulated industry, you are well-aware that it is a business-critical and ever-lasting journey. While you may be continuously on this journey, your colleagues, managers and auditors often want to get a quick overview about it. There are those who will want to understand the statistics […]
Risk Management in Jira: Streamlining the Process with Powerful Tools
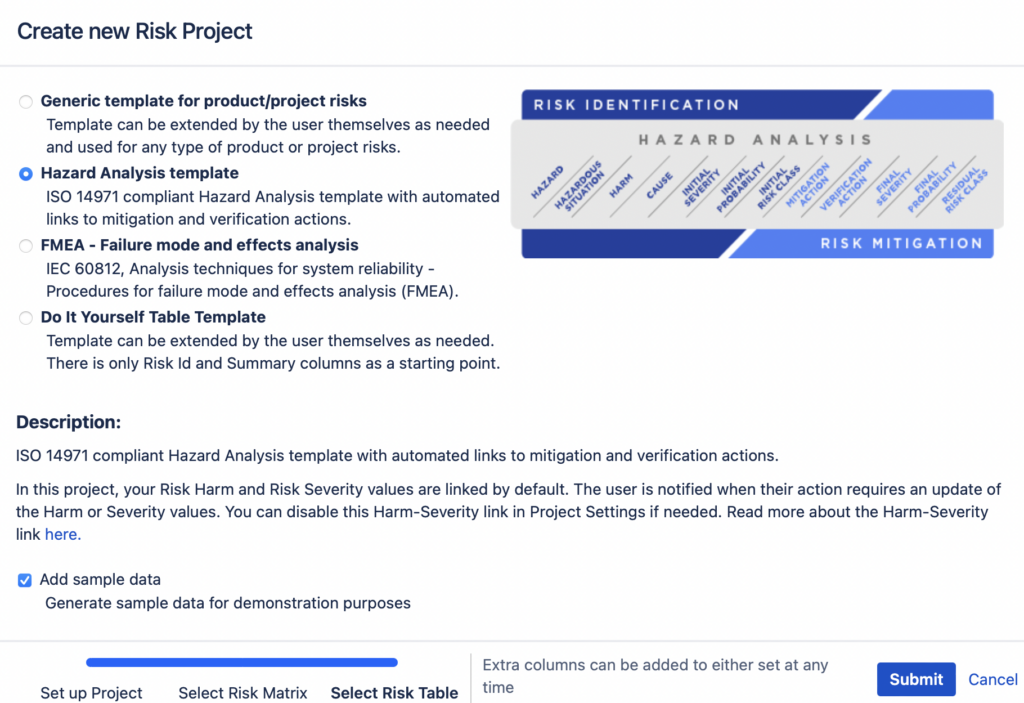
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a crucial role in ensuring project success and organizational stability. With the rise of digital solutions, integrating risk management practices into existing project management platforms has become a priority for many companies. In this article, we will explore how Jira, […]
