5 Steps To Tracking & Mitigating Project Risks in Jira
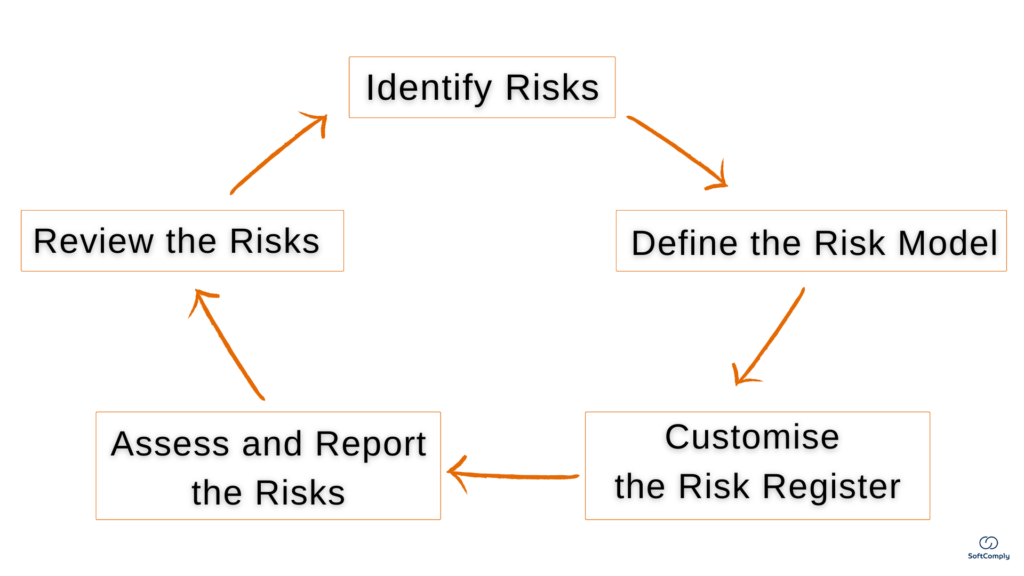
Project risk management is crucial for project success, especially important to keep critical risks under check in case of (inevitable) changes. The hidden complexities of a project is the main reason why timelines are not met and the resources are spent mid-way. Here is a quick guide for a Project Manager how to best conduct […]
Supporting Regulated Industries on Atlassian

On May 2, 2024 SoftComply hosted the 2nd edition of Regulated Industries workshop during Atlassian Team event. The workshop’s title was “How to best support Regulated Industries on Atlassian” and was aimed to discuss the customer feedback and best practices of collaboration between Atlassian, app vendors and solution partners to support our customers. For 2 […]
What are the FDA 21 CFR 11 Compliant Electronic Records?

Title 21 of the Code of Federal Regulations, Part 11, also known as 21 CFR 11, deals with the requirements for Electronic Records and Electronic Signatures to be considered “trustworthy” by the FDA. If you work in the MedTech or Pharma sector, you probably have heard about this regulation plenty of times. And if you […]
3 Best Jira Cloud Risk Management Plugins Compared [2024]

Comparison of risk apps on Jira Cloud
Don’t Leave Your Quality Team Behind!

Your Development team might find the instructions from Quality and Compliance team time-consuming – slowing down their delivery time, but the regulatory standards are put in place to ensure the safety, effectiveness and integrity of products from different industries. There are significant consequences for non-compliance ranging from loss of business and legal penalties to your products being […]
End App Fatigue with one Risk Management Solution

For decades organisations have been struggling with working in silos with different departments working with several stand-alone software tools, each for a specific task. With tools like Jira and Confluence that cater to a variety of different needs you can set up your entire organisation on one platform. So, there should be no more complaints? […]
HOW TO USE THE AUTOMATION ASSISTANT OF THE RISK MANAGER PLUS?

We have just released a new Automation Assistant for the SoftComply Risk Manager Plus app – a feature some of you have requested. We heard you and we have now delivered it 😉 With the Automation Assistant you can automatically set values to your risks without having to set them manually in the Risk Manager […]
How to Manage Information Security Risks in Jira in Compliance with ISO/IEC 27001

What is Information Security Risk Management? Information security risk management is a systematic process that involves identifying, assessing, prioritizing, and mitigating potential risks that could compromise an organization’s information assets. These assets may include customer data, intellectual property, financial information, or proprietary business processes. The risk management process allows companies to maintain confidentiality, integrity, and […]
Changes of Confluence Cloud in 2023: Detected by SoftComply

Confluence Cloud is constantly changing and not all the changes that are implemented by Atlassian are gated, i.e. they can’t be verified over the 2 weeks of Atlassian Release Tracks. SoftComply Validation app for Confluence Cloud is developed to monitor changes in and ensure the data integrity of your Confluence instance. The Validation app for […]
Increase your Efficiency in Confluence
Introducing the Toolbox App that Solves Three Critical Problems for Confluence Cloud Users Are you a Confluence user who has experienced the frustration of not being able to display page information in the body of a page, create static links to specific versions of pages, or delete pages and their children quickly and easily? If […]
