Hazard Analysis In Jira (Quick Guide)

If your team is working in Jira and you need to conduct hazard analysis, you can do so using the SoftComply Risk Manager Plus app. Hazard analysis is crucial in safety-critical product development, especially for medical devices. If you prefer to watch the video instead of reading, you can do so here. Key Takeaways: Understanding […]
How to use CVSS in Jira

What is CVSS and when to use it? The Common Vulnerability Scoring System (CVSS) is a standardized framework for rating the severity of security vulnerabilities. The CVSS was developed and is maintained by the Forum of Incident Response and Security Teams (FIRST). FIRST is an international consortium that aims to foster cooperation and coordination in […]
Don’t Keep a Risk Register in Excel (Use Jira)

If you’re considering using Excel for your risk register, this video explains why that might not be the best choice. Instead, we introduce a more effective alternative using Jira and dedicated risk management plugins. If you prefer watching over reading, check out the full video. Key Takeaways: What is a Risk Register The document where […]
Jira Risk Management Workflow Tutorial

In this article, we will look at the 4 steps needed to build a Risk Register in Jira. For those of you fluent in Jira, this tutorial will be focusing on risk lifecycle management not the native Jira issue workflows. What is a Risk Register and why create it in Jira? The document where an […]
3 Best Jira Risk Register Plugins for 2025

What is a Risk Register and Where to Build It? Risk Management is an essential governance practice for enterprise, product, portfolio, information security and project management. Proactive risk management ensures that the most significant risks that could impact the organization’s objectives are effectively mitigated. The document where an organization or a risk manager records all identified […]
Risk Manager: Roles & Responsibilities, Techniques & Trends

A risk manager continuously identifies, assesses, and mitigates potential risks that could impact an organization’s objectives. They develop strategies to minimize threats, ensure compliance with regulations, and protect company’s financial stability and reputation. Very often there is no one person called Risk Manager in an organisation. Risk management responsibilities are most typically shared between project […]
NIS2 and DORA Compliance and Protecting your Atlassian Cloud Data

This is a guest-article from Atlassian Marketplace Partner, Revyz In late 2022, the European Parliament introduced the Network and Information Systems Directive (NIS2) and the Digital Operational Resilience Act (DORA) to enhance cybersecurity across the EU. NIS2 requires compliance by EU member states by October 18th, 2024, focusing on robust security measures for digital service […]
Using Jira for Risk Management: 8 Best Practices For 2024

When it comes to managing risks in project management, using Jira for risk management can significantly improve your team’s productivity and effectiveness. Risk management involves identifying, assessing, and prioritizing risks to minimize their impact. With Jira’s flexible tools and plugins, you can streamline this process making it easier to keep track of potential issues and […]
5 Steps To Tracking & Mitigating Project Risks in Jira
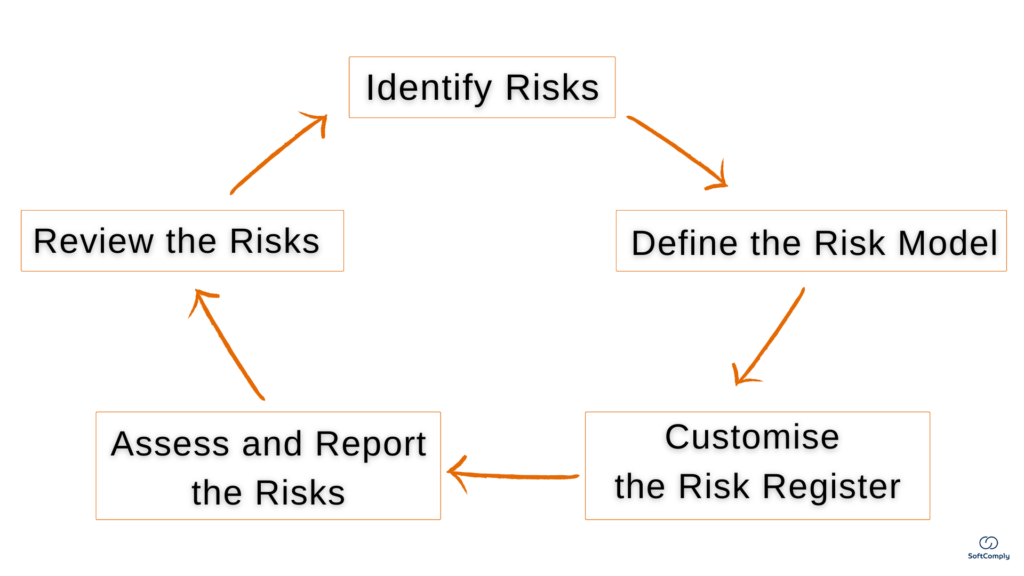
Project risk management is crucial for project success, especially important to keep critical risks under check in case of (inevitable) changes. The hidden complexities of a project is the main reason why timelines are not met and the resources are spent mid-way. Here is a quick guide for a Project Manager how to best conduct […]
Supporting Regulated Industries on Atlassian

On May 2, 2024 SoftComply hosted the 2nd edition of Regulated Industries workshop during Atlassian Team event. The workshop’s title was “How to best support Regulated Industries on Atlassian” and was aimed to discuss the customer feedback and best practices of collaboration between Atlassian, app vendors and solution partners to support our customers. For 2 […]
