Software Validation: A Comprehensive Approach to Software Validation in the Medical Device Industry

Automated Validation of Confluence Cloud
On the New FDA Guidance on Software Assurance

On September 13 2022, the FDA issued a new draft guidance on “Computer Software Assurance for Production and Quality System Software”. This new guidance is intended to supplement the current approach described in the 2002 “General Principles of Software Validation” guidance. This guidance additionally discusses specific risk considerations, acceptable testing methods, and efficient generation of […]
How to Maintain a Documentation Baseline of Agile Development in Confluence?

most popular use cases for capturing and freezing the always dynamic data in Confluence with the SoftComply Static Snapshots app
Change Management & Medical Device QMS on Confluence

How to keep your QMS up to date when regulations and standards change over time with the help of a Compliance Matrix
Display Document Approvals on Confluence Pages with the SoftComply Change History app

display your document approval history on Confluence pages with the SoftComply Change History app
Introducing the Cloud eQMS Solution for Confluence

Why Confluence? Or perhaps the first question should be – why quality management? Most medical device companies need to have a compliant quality management system in place to assure consistent quality of their product. Quality management system consists of a number of written procedures and a multitude of records that will be generated during the […]
How to Create a Risk Traceability Matrix in Jira
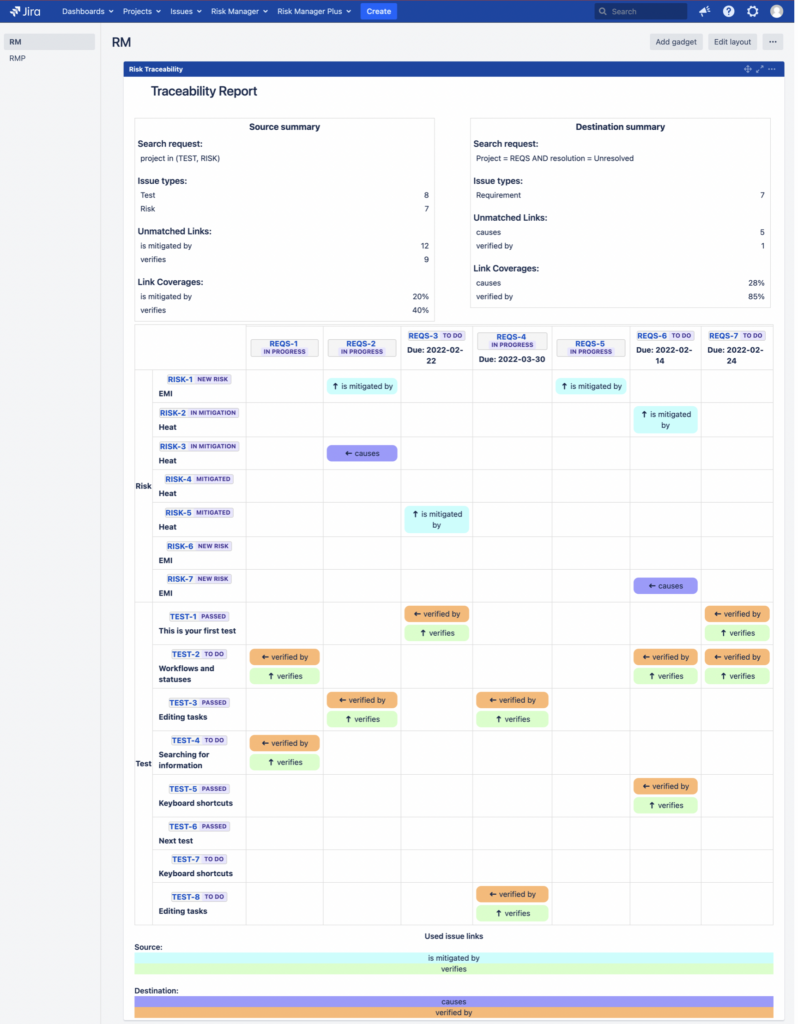
Introduction Risk Traceability is a great way to get a quick overview of the completeness of risk management process and it is often used in the regulated industries. The traceability matrix shows the coverage of risks by mitigation and verification actions. For example, in the medical device industry, both the FDA and ISO 14971 require bi-directional […]
Risk Management in Jira: Streamlining the Process with Powerful Tools
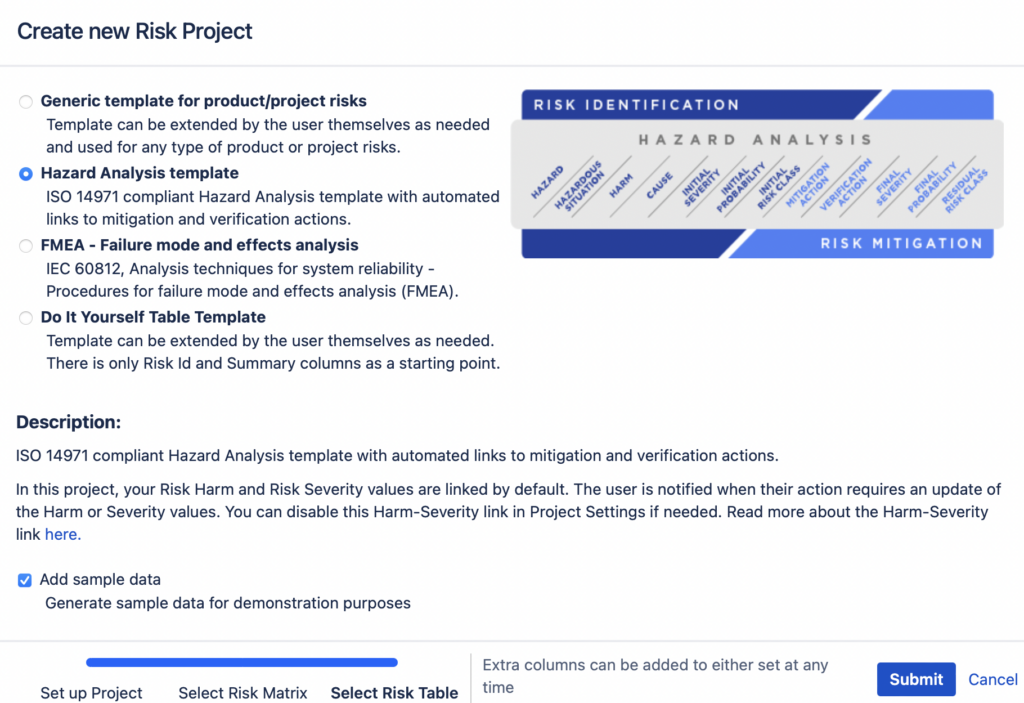
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a crucial role in ensuring project success and organizational stability. With the rise of digital solutions, integrating risk management practices into existing project management platforms has become a priority for many companies. In this article, we will explore how Jira, […]
How to Make your Confluence Pages Static & Compare them with the SoftComply Static Snapshots app

Introduction With the SoftComply Static Snapshots app you can capture and freeze the dynamic content of your pages in Confluence Cloud. This is something you may want to do to better measure progress over time or to make sure that the content of the pages that need to be approved will not change in the […]
Six Must-Have Atlassian Cloud Apps for the Regulated Industries
Introduction With Atlassian’s Server end of life approaching in a few years, most companies need to decide whether to migrate to Cloud or to Data Center. Data Center may seem like a natural path to take for the larger (over 300 users) companies in the regulated domains. Nevertheless, there are some regulated industries like the […]
