In Search of a Compliance Solution that Lets You Sleep at Night

How to find the best regulatory compliance solution for medtech on Atlassian
Software Validation: A Comprehensive Approach to Software Validation in the Medical Device Industry

Automated Validation of Confluence Cloud
On the New FDA Guidance on Software Assurance

On September 13 2022, the FDA issued a new draft guidance on “Computer Software Assurance for Production and Quality System Software”. This new guidance is intended to supplement the current approach described in the 2002 “General Principles of Software Validation” guidance. This guidance additionally discusses specific risk considerations, acceptable testing methods, and efficient generation of […]
How to Maintain a Documentation Baseline of Agile Development in Confluence?

most popular use cases for capturing and freezing the always dynamic data in Confluence with the SoftComply Static Snapshots app
Change Management & Medical Device QMS on Confluence

How to keep your QMS up to date when regulations and standards change over time with the help of a Compliance Matrix
Display Document Approvals on Confluence Pages with the SoftComply Change History app

display your document approval history on Confluence pages with the SoftComply Change History app
SOFTCOMPLY RISK MANAGER IS NOW CLOUD FORTIFIED

Introduction Last week we reached an important milestone – the SoftComply Risk Manager on Jira Cloud was awarded the Cloud Fortified badge by Atlassian. The SoftComply Risk Manager is the only product risk management app on Jira and the most customisable one with Risk Management guidance, multiple views to work in and compliant Risk Reporting. To learn more about the SoftComply Risk Manager on Jira Cloud, please book your […]
Introducing the Cloud eQMS Solution for Confluence

Why Confluence? Or perhaps the first question should be – why quality management? Most medical device companies need to have a compliant quality management system in place to assure consistent quality of their product. Quality management system consists of a number of written procedures and a multitude of records that will be generated during the […]
How to Create a Risk Traceability Matrix in Jira
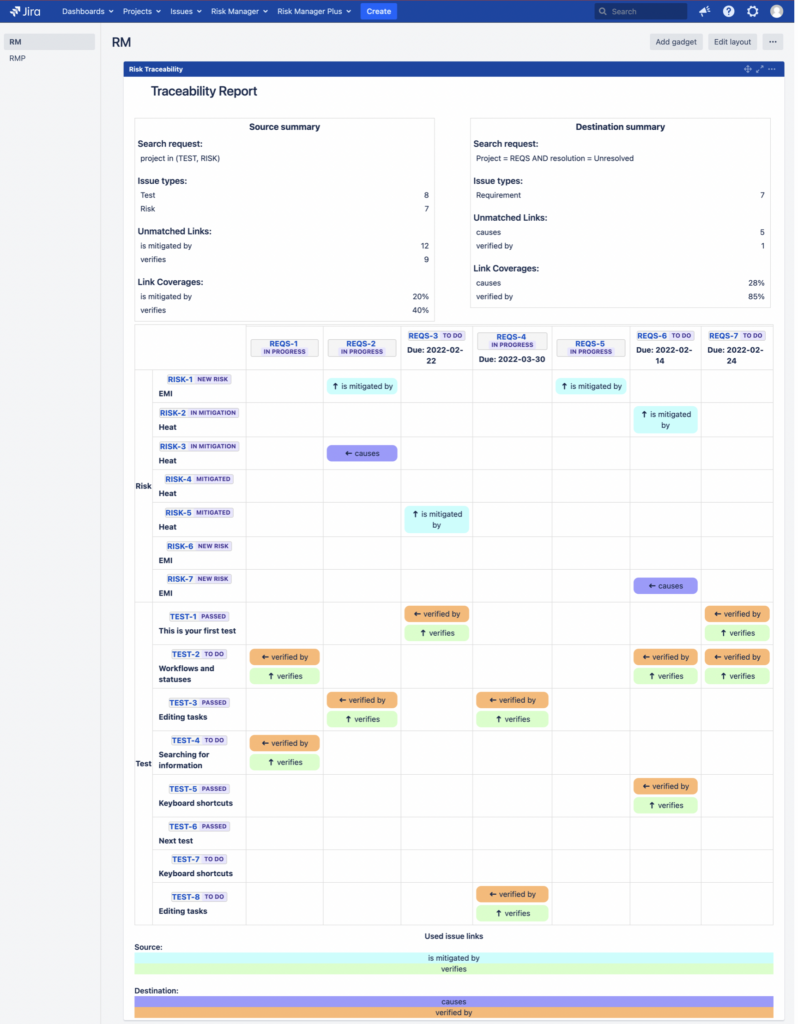
Introduction Risk Traceability is a great way to get a quick overview of the completeness of risk management process and it is often used in the regulated industries. The traceability matrix shows the coverage of risks by mitigation and verification actions. For example, in the medical device industry, both the FDA and ISO 14971 require bi-directional […]
How to Create a Risk from a Jira Support Ticket?
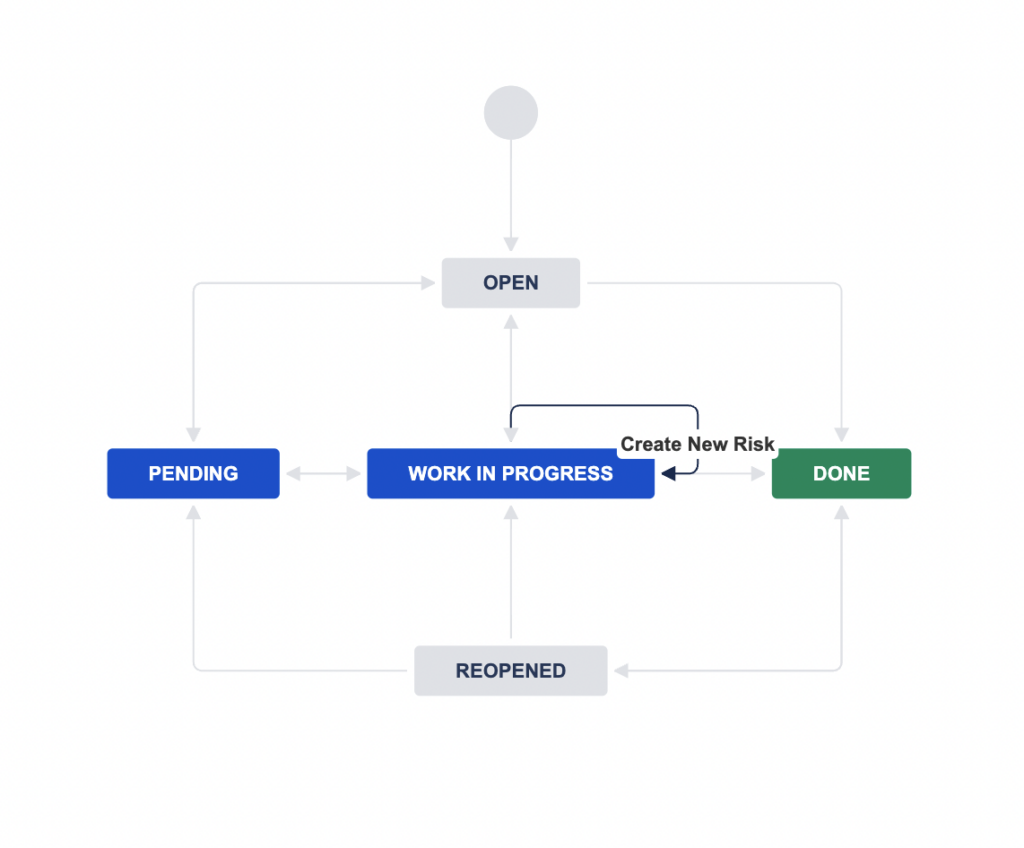
Introduction Jira is the most flexible task management tool there is. No wonder that more than 200’000 companies across the world have found it useful. It helps software teams build their products, prioritise their backlog and manage any upcoming issues within their team. Most of these companies are using Jira Service Management to collect customer […]
