Display Document Approvals on Confluence Pages with the SoftComply Change History app

display your document approval history on Confluence pages with the SoftComply Change History app
SOFTCOMPLY RISK MANAGER IS NOW CLOUD FORTIFIED

Introduction Last week we reached an important milestone – the SoftComply Risk Manager on Jira Cloud was awarded the Cloud Fortified badge by Atlassian. The SoftComply Risk Manager is the only product risk management app on Jira and the most customisable one with Risk Management guidance, multiple views to work in and compliant Risk Reporting. To learn more about the SoftComply Risk Manager on Jira Cloud, please book your […]
How to Report Risks in Jira
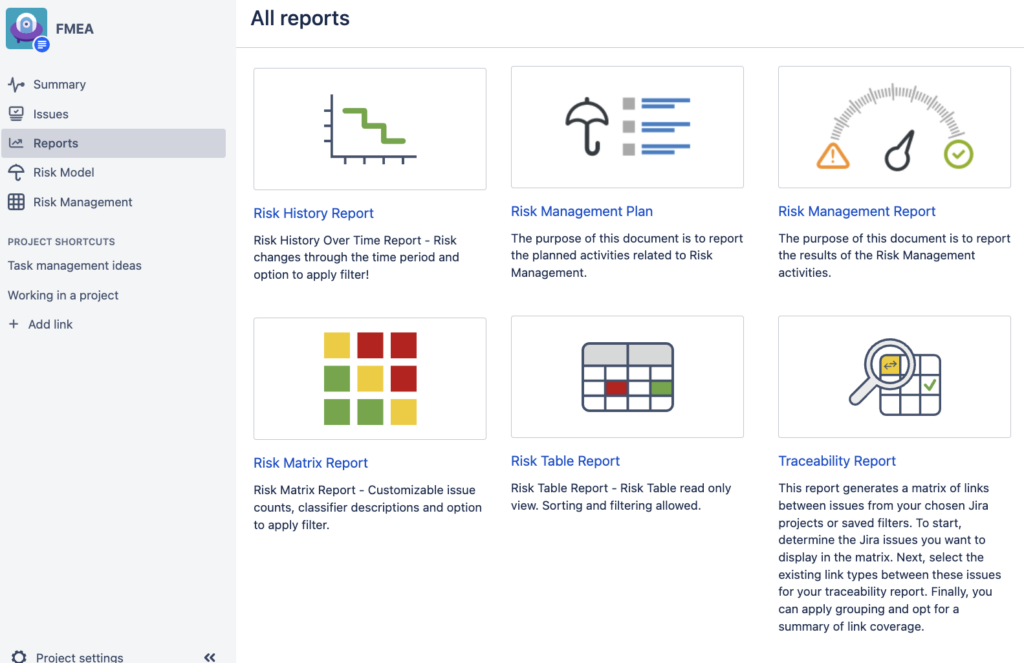
Introduction When you manage your product risks in a highly regulated industry, you are well-aware that it is a business-critical and ever-lasting journey. While you may be continuously on this journey, your colleagues, managers and auditors often want to get a quick overview about it. There are those who will want to understand the statistics […]
Risk Management in Jira: Streamlining the Process with Powerful Tools
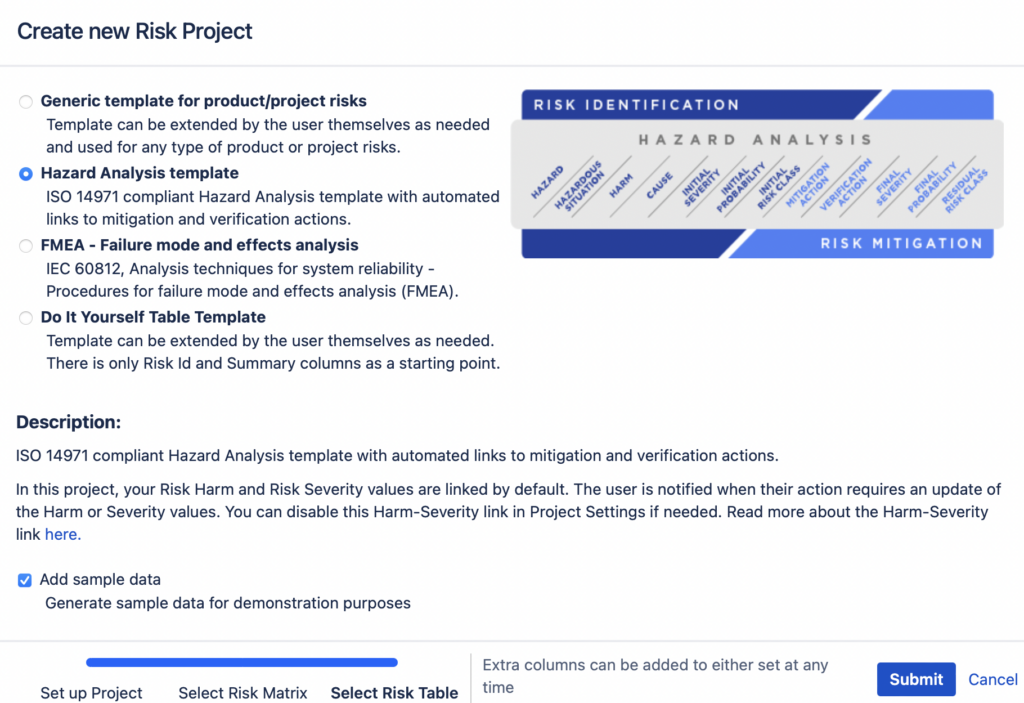
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a crucial role in ensuring project success and organizational stability. With the rise of digital solutions, integrating risk management practices into existing project management platforms has become a priority for many companies. In this article, we will explore how Jira, […]
How to Make your Confluence Pages Static & Compare them with the SoftComply Static Snapshots app

Introduction With the SoftComply Static Snapshots app you can capture and freeze the dynamic content of your pages in Confluence Cloud. This is something you may want to do to better measure progress over time or to make sure that the content of the pages that need to be approved will not change in the […]
How to Report Risks in Confluence
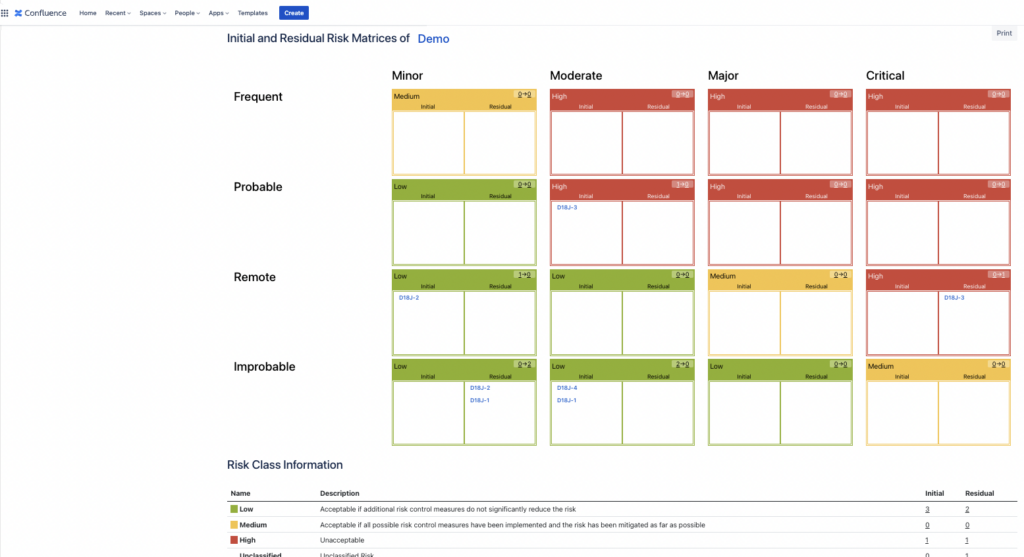
Introduction Managing your product risks in Jira is great – you can use the flexibility of Jira to add various fields to the risk table, customise the risk table and the risk matrices and filter/sort the data any which way you want when using the SoftComply Risk Manager app on Jira. But what if you’d like to report your […]
Six Must-Have Atlassian Cloud Apps for the Regulated Industries
Introduction With Atlassian’s Server end of life approaching in a few years, most companies need to decide whether to migrate to Cloud or to Data Center. Data Center may seem like a natural path to take for the larger (over 300 users) companies in the regulated domains. Nevertheless, there are some regulated industries like the […]
How to Manage Requirements in Confluence and Jira

Introduction to Requirements Management in Jira and Confluence Requirements management in the regulated domains such as medtech, automotive or aviation has certain differences compared to the agile development. Jira Software with its easy to use backlog view and work boards suits best for managing requirements in the agile world. For the regulated domains it might […]
How to keep your data in Confluence static?
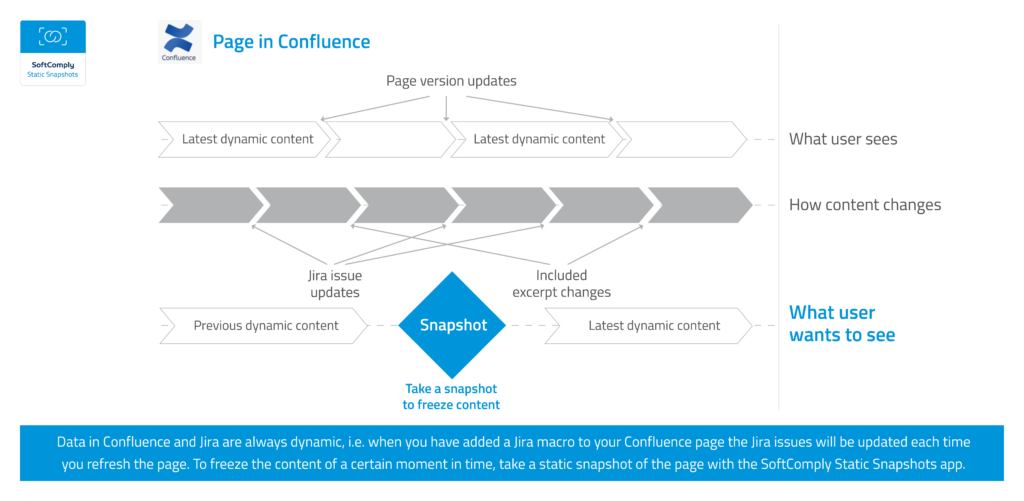
If you have worked with Jira and Confluence, am sure you have enjoyed the integration between the two. The way you can easily pull data from Jira to Confluence by creating macros on your Confluence page with all the data from Jira that you want to display is absolutely wonderful. Now, every time you refresh […]
Relationship between FDA & ISO 13485

The relationship between the FDA and ISO 13485 goes back a long time. The ISO standard has been in the list of General Consensus Standards since the dawn of times, but there has never been a full alignment between 21 CFR 820 (QSR) and ISO 13485. It’s like these couples that have been together forever, […]
