How to Manage Requirements in Confluence and Jira

Introduction to Requirements Management in Jira and Confluence Requirements management in the regulated domains such as medtech, automotive or aviation has certain differences compared to the agile development. Jira Software with its easy to use backlog view and work boards suits best for managing requirements in the agile world. For the regulated domains it might […]
How to keep your data in Confluence static?
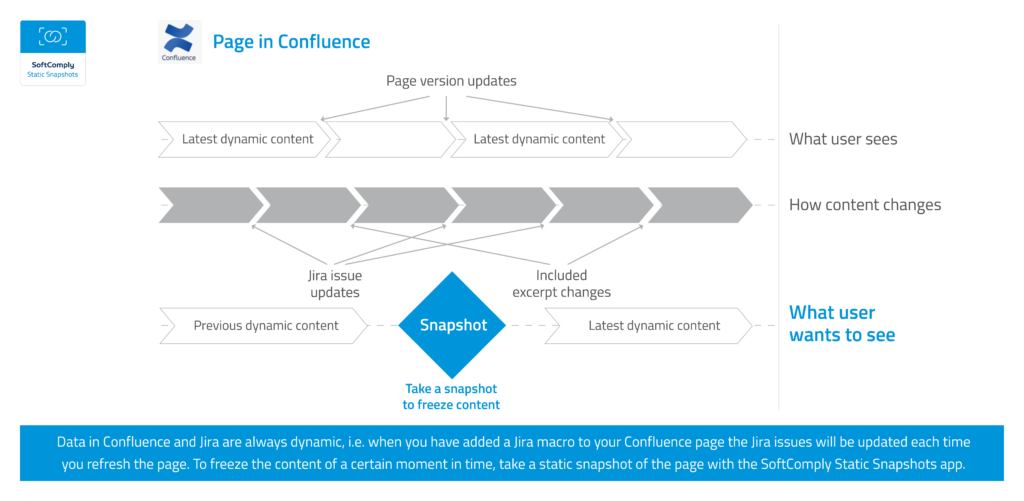
If you have worked with Jira and Confluence, am sure you have enjoyed the integration between the two. The way you can easily pull data from Jira to Confluence by creating macros on your Confluence page with all the data from Jira that you want to display is absolutely wonderful. Now, every time you refresh […]
Risk Management Guide for a Digital Health or a Medical Device Company on Jira Cloud
Why Risk Management In the fast-paced world of medical device development, safety and quality are of utmost importance. Manufacturers must adhere to stringent regulations and standards to ensure that their devices are safe and effective for patients. One such standard that plays a crucial role in the medical device industry is ISO 14971, which focuses […]
How to Build a Risk Analysis in Jira

How to build a risk analysis in Jira with the SoftComply Risk Manager app
Compliance of myBioma

The Regulatory Compliance Journey of myBioma Biome Diagnostics GmbH is an Austrian medtech start-up utilizing the genetic information of the microbiome and AI to develop medical diagnostic software for doctors. Biome Dx products utilize technologies such as next-generation sequencing, pipeline architectures and microservices. With their lifestyle product myBioma every European has the possibility to learn […]
3 MUST-HAVE AREAS TO CONSIDER WHEN SETTING YOUR ORGANIZATION UP ON ATLASSIAN STACK

3 things regulated industries must consider when setting up their organisations on Atlassian Jira and Confluence
How to apply SoftComply Risk Manager features to any Jira project
Wouldn’t it be cool to keep everything in Jira – your backlog, your test cases and your risks all in Jira? Yes, but you could do that for years already. But what if all that can be done in a single Jira project? That would be truly awesome! You can now do it with the […]
How to Add Page Numbers to the PDF Exports of Quality Records in Confluence
Paper-based Quality Management Systems are now (almost) a thing of the past (it’s sooo 20th century…). But in some cases, printing records and documents from the eDMS is necessary, or at least exporting them to PDF. The requirements for paper documents are the same as digital documents. But, unlike electronic formats, physical misplacement or loss […]
Medical Device Compliance & Atlassian Cloud
On the 16th of October 2020, Atlassian announced radical changes to their offering. From February 2nd 2021, Atlassian will not sell any new Server licenses for any of their products. A year later, in February 2022, it will not be possible to upgrade or downgrade your Atlassian Server product tier. From February 2023, it will […]
How We Managed the Compliance of Personal Protective Equipment

The Regulatory Compliance Journey of XRGO Nathaniel Victor, CEO of XRGO: “We got involved in PPE manufacturing as a goodwill donation/gesture of time, energy, products and financial support to the hospitals and local municipalities. Early on, our suppliers in China informed us that COVID-19 was serious and they advised that we protect our staff and […]
