If your team is working in Jira and you need to conduct hazard analysis, you can do so using the SoftComply Risk Manager Plus app. Hazard analysis is crucial in safety-critical product development, especially for medical devices. If you prefer to watch the video instead of reading, you can do so here.
Key Takeaways:
- Hazard analysis helps assess potential hazards, hazardous situations and possible harms of safety-critical products.
- It is a requirement for medical device developers as per ISO 14971.
- The SoftComply Risk Manager Plus app provides a list of hazards and allows customization for specific devices.
- The app supports risk assessment by offering risk model and risk register templates for hazard analysis, and it ensures traceability between requirements, risks, and tests.
- Users can try the SoftComply Risk Manager Plus app for free for one month on Jira Cloud.
Understanding Hazard Analysis
Hazard analysis plays a pivotal role in ensuring the safety and reliability of products, particularly in safety-critical industries like medical devices. Leveraging tools like Jira and the SoftComply Risk Manager Plus app can streamline this process, making it efficient and comprehensive. This guide will walk you through the essentials of hazard analysis and how to master it using Jira with the Risk Manager Plus app.
Definition: Hazard analysis is the systematic process of identifying, evaluating, and controlling potential hazards that could cause harm in the event of product malfunction. In essence, it’s about pinpointing what could go wrong and how to prevent it, especially in high-stakes environments like medical technology.
Components: The essential elements of hazard analysis include:
- Hazard Identification: Spotting potential problems that could arise from the product’s use.
- Risk Assessment: Assessing the severity and probability of each identified hazard.
- Risk Control: Implementing measures to mitigate or eliminate the identified risks.
- Traceability & Reporting: Keeping detailed records to ensure traceability and compliance with regulatory requirements.
Importance: The significance of hazard analysis cannot be understated, particularly in medical device development. Lives can literally be on the line. A rigorous hazard analysis process ensures that potential risks are identified and managed before they result in harm. It’s not just about compliance; it’s about creating a safer, more reliable product.
ISO 14971 Standard
ISO 14971 is an internationally recognised standard for risk management in the development of medical devices. It provides a systematic framework for identifying, evaluating, and mitigating the risks associated with medical devices throughout their lifecycle. Understanding this standard is critical for ensuring product safety and regulatory compliance.
Overview
At its core, ISO 14971 outlines the principles and processes for risk management, including risk analysis, risk evaluation, risk control, and monitoring the effectiveness of the risk controls. It is designed to help manufacturers ensure that medical devices are as safe as possible, thereby protecting patients and users.
Relevance
ISO 14971 standard is crucial in guiding the hazard analysis process within the medical device industry. It specifies that manufacturers must identify potential hazards, estimate and evaluate the associated risks, implement control measures, and continuously monitor the effectiveness of these measures. This iterative process helps in maintaining a high level of safety and efficacy in medical devices.
SoftComply Integration
The SoftComply Risk Manager Plus app is built to streamline compliance with ISO 14971 standard. It incorporates the guidance and hazard management principles outlined in ISO 14971, making it easier for teams to follow a standardised approach. The app includes risk model and risk register templates for hazard analysis as well as a pre-built list of hazards from ISO 14971, facilitating quick and compliant hazard analysis. By integrating this standard within Jira, the Risk Manager Plus app helps ensure that your risk management process is efficient and compliant.
Risk Assessment
When diving into the risk assessment, it’s crucial to break the process down into manageable chunks. The main steps involve evaluating risks based on both severity and probability. Here’s how you can master this process using Jira with the SoftComply Risk Manager Plus app:
First off, the risk assessment process starts with listing potential risks. Once these risks are identified, you’ll assess them based on their severity (how bad would it be if it happened?) and probability (how likely is it to happen?). Each risk is rated on these axes to help prioritize what needs the most attention.
Traceability is paramount. You need to keep a clear link between requirements, risks, and tests. This means that each piece of the puzzle should be traceable back to its origin, ensuring nothing falls through the cracks. SoftComply’s app makes this straightforward by allowing seamless linking of risks to requirements (risk controls) and subsequently to tests (verification of risk controls). It’s all about creating a traceable chain that enhances accountability and clarity.
Practical Steps
Here’s your step-by-step approach to conducting risk assessment in Jira:
- Start with Setup: Ensure the Risk Manager Plus app is installed and properly configured in your Jira instance.
- List Risks: Begin by listing all potential risks associated with your product directly within the app.
- Evaluate Severity and Probability: For each risk, determine its severity and probability using the built-in scoring system. This gives you a numerical foundation to prioritize.
- Link Controls to Risks: Use the issue linking to add a risk control to a risk. This helps in maintaining an organized structure and makes sure every critical risk has a control measure.
- Trace to Tests: Once risk controls are linked to risks, map the relevant test cases to them as well to indicate that the control measures work as expected.
By following these steps, you’ll be able to conduct a comprehensive risk assessment. The process is streamlined thanks to SoftComply Risk Manager Plus, which doesn’t just save you time but also supports your product’s safety.
Features of the SoftComply Risk Manager Plus
Templates for Risk Models and Tables
Risk Manager Plus comes with a selection of templates designed to get you up and running quickly. These templates cover a variety of risk models and tables, allowing you to customize them to your specific project needs. Whether you’re working on a new medical device or updating an existing product, these templates can save a ton of time and effort.
Linking Risks, Requirements and Tests
One of the standout features of Risk Manager Plus is its ability to link risks with controls (requirements) and verification actions (tests) seamlessly in the risk table view. This linking functionality ensures that every identified risk is traceable to a control measure which has been tested. It simplifies review processes and helps maintain consistency across the project lifecycle.
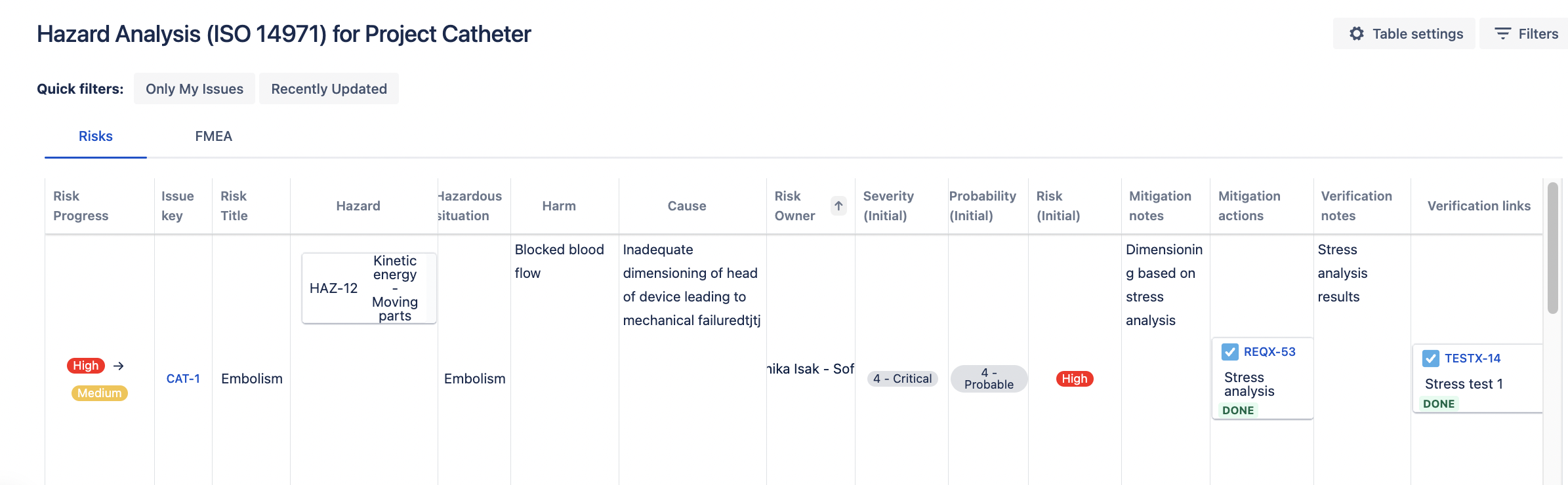
Benefits:
- Traceability: Linking risks, requirements and tests for establishing full traceability.
- Simplified Reviews: Easier audits and review processes.
- Consistency: Maintains uniformity across the project lifecycle.
Ready-Made List of Hazards
The Risk Manager Plus app includes a pre-built list of common hazards, which can be a huge time-saver. This list incorporates industry standards and best practices, meaning you have a solid foundation right from the start.
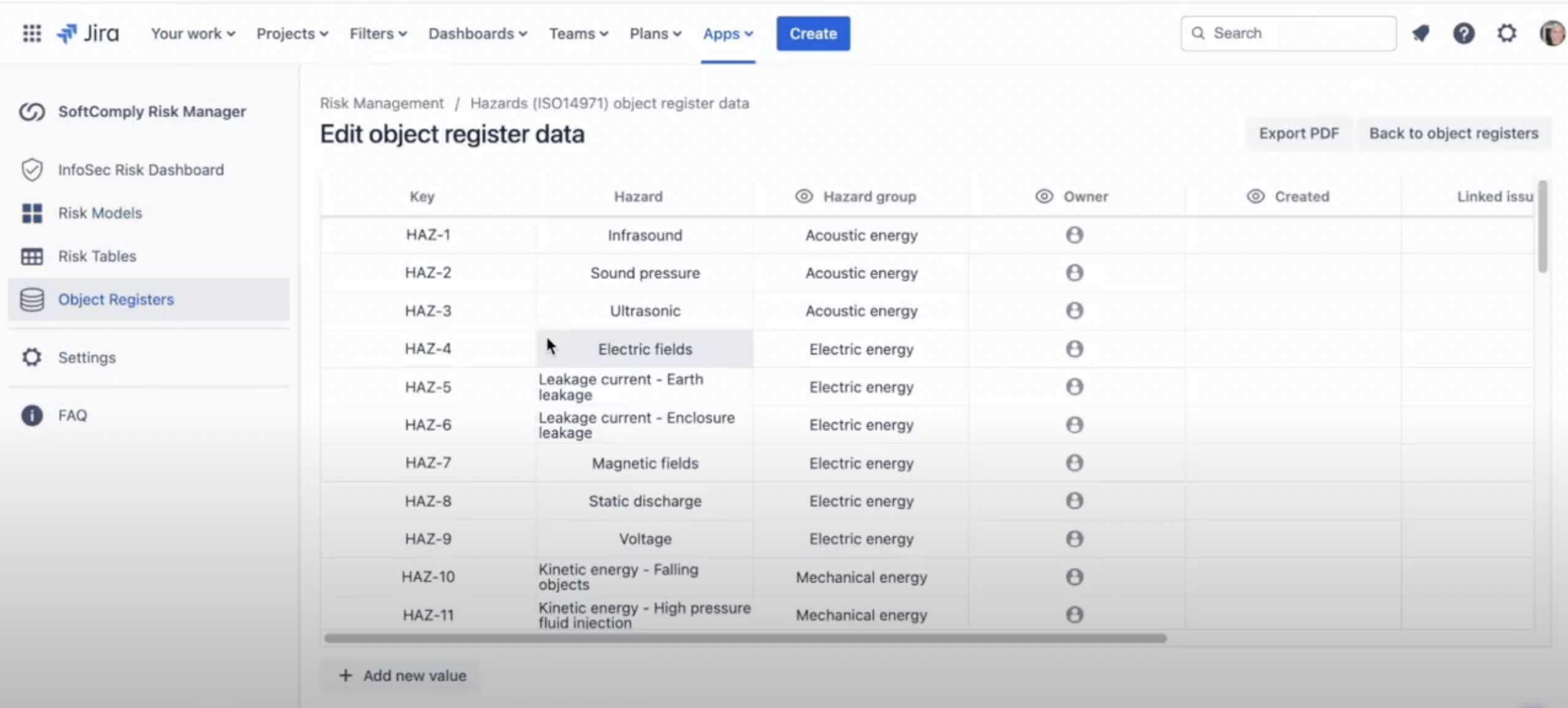
Advantages:
- Quick Identification: Fast track the identification of potential hazards.
- Error Reduction: Minimize manual entry errors.
- Comprehensive Coverage: Ensure all potential hazards are considered.
Using the ready-made list, you can quickly identify potential hazards and integrate them into your risk analysis. This reduces manual entry errors and ensures you’re covering all bases.
Mastering Hazard Analysis in Jira with Risk Manager Plus
Step-by-Step Guide
- Setup:
Start by installing the Risk Manager Plus app in your Jira instance. Navigate to Atlassian Marketplace, search for “SoftComply Risk Manager Plus”, and click Try it out. You can try out the app for free for 30 days.
Follow the prompts to get the app up and running. This foundational step ensures that you’re equipped with the essential tools needed to streamline hazard analysis in Jira. - Choose or Create a Risk Model:
With the app installed, you can create your first risk model or choose one from the templates provided. Go to the Risk Manager Plus dashboard and select “Create New Risk Model.” Utilize the pre-built templates available, which you can customize according to your project specifications. Enter details such as risk categories, severity and likelihood, tailoring the model to fit the specific needs of your product development lifecycle. - Linking Risks, Requirements and Tests: Establishing traceability between risks, requirements and tests is crucial for ISO 14971 compliance. Within the Risk Manager Plus app, you can associate each risk with relevant product requirements and tests in the Risk Table view. This linking helps ensure comprehensive traceability, i.e. all risks have control measures in place.
- Conducting Risk Assessment:
Perform detailed risk assessments by evaluating the severity and probability of each identified hazard. The app allows you to visualize these parameters easily, using risk matrices and dashboards to manage and prioritize risks effectively. Ensure you add mitigation strategies for high risks. - Using the Hazards List:
The SoftComply Risk Manager Plus app comes with a handy pre-built hazards list. This feature is a significant time-saver. Access the “Hazards List” from the Object Register module and browse through the comprehensive collection of common hazards associated with your device. Select appropriate hazards to include them in your risk assessment. This systematic approach minimizes the likelihood of overlooking potential issues.
Best Practices
- Consistency: Maintain a consistent format and structure for all risk-related documentation. Ensure that risk records are complete and uniformly structured across different projects for better reliability and efficiency.
- Regular Updates: Make it a routine to revisit and update your risk assessments and hazard analyses regularly. The dynamic nature of product development means that new risks can emerge at any stage, and current risks might evolve over time. You can use notifications and automation for reminding risk reviewers to periodically review risks.
- Training: Conduct regular training sessions for your team to ensure they are proficient in using Jira and the Risk Manager Plus app.
By following these steps and integrating best practices, you’ll be well on your way to mastering hazard analysis in Jira with SoftComply’s Risk Manager Plus app. This structured approach not only optimizes the efficiency and thoroughness of your risk management processes, but also helps ensure your products meet stringent safety standards. For further reading and to enhance your product safety with FMEA, please check out our recent blog post on how hazard analysis and FMEA relate to each other.
To Recap:
- Hazard Analysis Essentials: Understand what hazard analysis entails and its critical role in safety-critical product development.
- ISO 14971 Guidance: Get acquainted with ISO 14971 and how SoftComply Risk Manager Plus app on Jira Cloud aligns with it.
- Risk Assessment Process: Learn to evaluate risks based on severity and probability while maintaining traceability.
- Risk Manager Plus Features: Utilize templates, linking functionalities, and pre-built hazards lists to simplify your workflows.
- Step-by-Step Mastery: Follow our detailed guide to setting up and mastering hazard analysis in Jira with Risk Manager Plus.
Now, it’s your turn. Implement these steps and best practices to elevate your hazard analysis game. Stay consistent with your documentation, regularly update your assessments, and ensure your team is well-trained on these robust tools.
👉 Try SoftComply Risk Manager Plus free for a month: https://marketplace.atlassian.com/apps/1219692/softcomply-risk-manager-plus-top-risk-management-in-jira?tab=overview&hosting=cloud
👉 Book a live demo: https://calendly.com/softcomply/softcomply-risk-manager-demo





