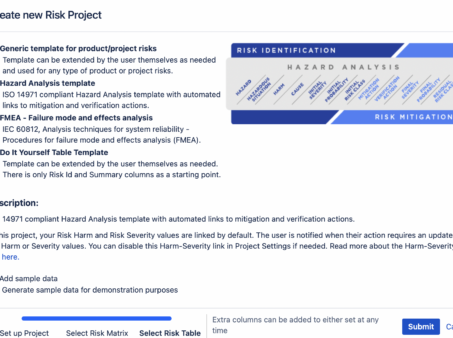
Risk Management in Jira: Streamlining the Process with Powerful Tools
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a…
SoftComply
Comparison of Risk Management Apps on Jira Cloud
Following our previous post where we compared the 4 top risk management apps on Jira Server,…
SoftComply
What are the best Risk Management Apps on Jira & how they compare to each other?
In the following post we compare the features of 4 top risk management apps on Jira…
SoftComply
What is a Risk/Benefit Analysis & How To Do It?
The Risk / Benefit analysis is one of the most misinterpreted areas of the Risk Management…

What is FMEA and how is it different from Hazard Analysis?
From ISO 14971: “FMEA is a technique by which the consequences of an individual fault mode…
SoftComply
New Medical Devices Regulation & Risk Management
The revised Medical Devices Regulation (MDR) will change the regulatory environment of medical devices in Europe…
SoftComply
What is FMEA and when to use RPN?
Per ISO 14971, “Failure Mode and Effects Analysis (FMEA) and Failure Mode, Effects and Criticality Analysis…
SoftComply
How I came to hate Excel & decided to develop an automated Risk Management tool for JIRA
Part II By Matteo Gubellini, VP of Regulatory Affairs at SoftComply* So you are looking for…
SoftComply
How I came to hate Excel & decided to develop an automated Risk Management tool for JIRA
Part I By Matteo Gubellini, VP of Regulatory Affairs of SoftComply* “Ok, let’s follow a few…