Customer Story on the MediCompli Solution at Cibiltech
NOTE: Cibiltech has now successfully migrated from Atlassian Server to Atlassian Cloud and have implemented the Cloud eQMS Solution as their Quality Management Solution.
Cibiltech
Cibiltech is a French medical device company developing an AI based personalized medicine solution for better patient care. Their most recent solution is aimed at post-transplant kidney monitoring for personalized treatment and better decision support.
Previous Document Management System at Cibiltech
Prior to the MediCompli Soluton, Cibiltech used Asana to link documents that were kept in Google Drive. Approved versions of quality system documents were signed by Adobe Sign and saved in pdf format in Google Drive.
What were the Requirements for Compliant Document Management System?
Cibiltech had the following requirements for their compliant Document Management System:
-
- Cibiltech was using Jira in their own development and one of the most important requirements were to have a seamless integration between development projects and requirements in Jira and Quality System documents;
- As Cibiltech’s products are for both EU and the US markets, compliance with both FDA (specifically FDA 21 CFR 11) and MDR requirements was paramount;
- As Cibiltech is developing Software as a Medical Device, they wanted to have a solution that is best fit for medical device software development;
- As their previous Document Management System required using various software tools, they wanted to have one single solution for compliant Document Management System;
- After a demo session, they also wanted to spend a few weeks trying out the functionality of the offered Document Management System themselves.
Why was the MediCompli Solution best fit for Cibiltech?
The MediCompli Solution ticked all the boxes for Cibiltech meeting all their requirements:
-
- Cibiltech spent weeks trialling the MediCompli Solution to fully experience the functionality of the solution;
- Since the MediCompli solution is native to Atlassian Confluence, Cibiltech enjoyed the seamless integration between their development requirements and risks in Jira and the quality system documents of the MediCompli solution in Confluence;
- The MediCompli solution is the most affordable fully FDA 21 CFR 11 compliant Document Management System out there;
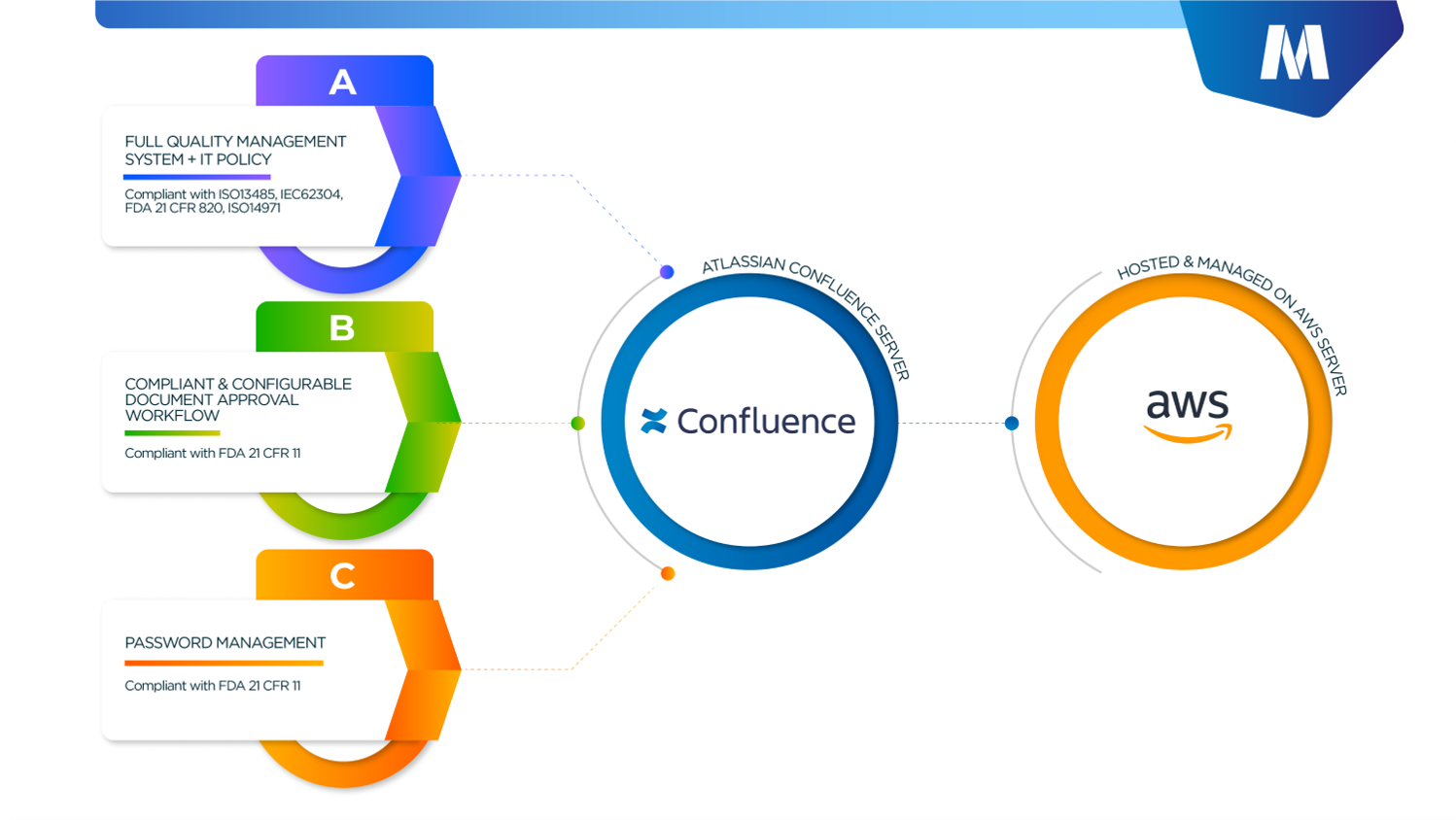
- The MediCompli solution is fully customizable and highly configurable as a Confluence native solution with QMS documentation based on IEC62304 requirements and therefore best fit for medical device software development projects.
Following table illustrates Cibiltech’s experience with and comparison of the MediCompli vs Greenlight Guru, the MediCompli vs MasterControl, and the MediCompli vs Aligned Elements:
What are the Best Features of the MediCompli Solution according to Cibiltech?
The features of the MediCompli Solution that Cibiltech like the best:
-
- Seamless integration to Jira and Bitbucket as the MediCompli solution is native to Confluence;
- The MediCompli solution comes with a full Quality System documentation based on ISO 13485, IEC 62304, ISO 14971 & FDA 21 CFR 820;
- Cibiltech also enjoyed the quality of the demo and the fast support;
- Cibiltech trialled the MediCompli solution prior to implementing it;
- The MediCompli solution comes with training and support to get all users on board quickly.
Learn more about the MediCompli Solution, the only FDA 21 CFR 11 and MDR Compliant Document Management System on Confluence
Check out more information about the MediCompli Solution, check out the MediCompli demo recording or arrange an online demo call with us!