Introduction to Requirements Management in Jira and Confluence
Requirements management in the regulated domains such as medtech, automotive or aviation has certain differences compared to the agile development. Jira Software with its easy to use backlog view and work boards suits best for managing requirements in the agile world. For the regulated domains it might be better to use Confluence in addition to Jira. In this blog post, we will describe how to use Confluence and Jira for requirements management in the regulated domains.
The main characteristic of agile development is, unsurprisingly, agility. While you are getting all ideas about and feedback to your backlog you are continuously prioritising and managing the backlog. In other words, your requirements are not fully analysed and may still change during the development phase. In the regulated domains, however, requirements need more control. Changes to requirements have to be analysed and follow a defined process of revision and approval by specific parties within the organisation. This is why the requirements for the requirement management systems differ based on the domain you are working in.
There are some advantages of using Confluence for the requirements management in the regulated domain compared to Jira. In Confluence:
- you can easily see the change history by using Confluence page versions diffs;
- it is easier to write requirements in. Adding images, diagrams, macros, etc and using wysiwyg editor is more user-friendly than the Jira issue editor;
- Confluence page templates help adding control over your content;
- you can add compliant doc approvals to Confluence pages with apps from Comala portfolio;
- requirements can be exported easily into different formats with different styles as needed;
- requirements are all in one place with easy to use structure. Organising and grouping requirements is easy inside a Confluence space. Just drag and drop to change the structure;
- it is easy to differentiate drafts and approved documents. Keep drafts and approved documents in separate spaces with separate access permissions.
In case the above-listed aspects are important to your organisation, then you will benefit from using Confluence to manage the requirements.
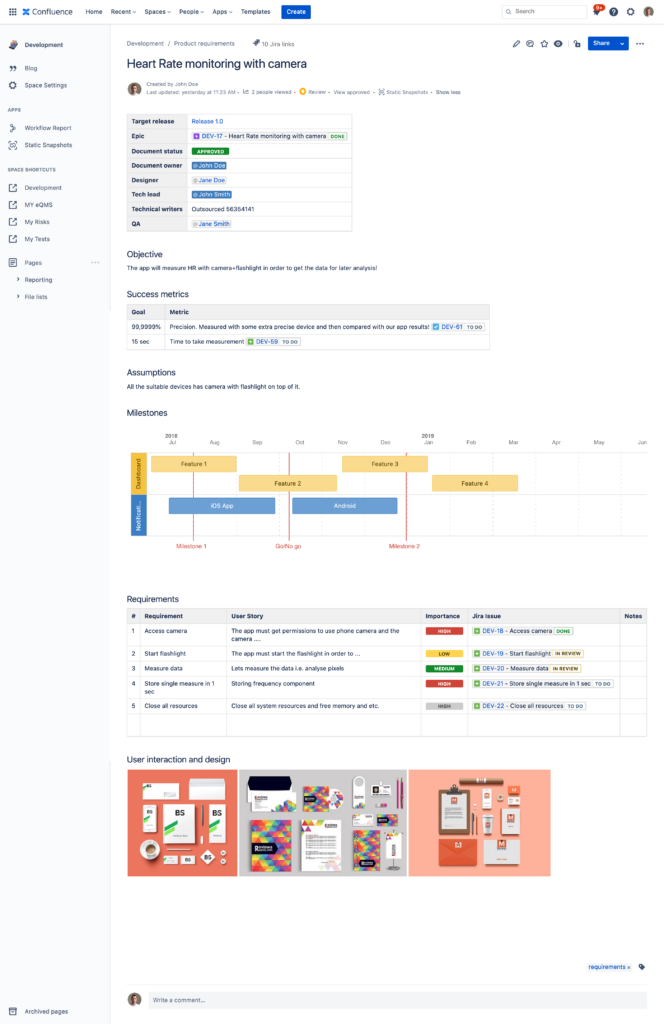
How to do Roadmapping and Release Planning when Requirements are in Confluence?
You can still do all of that in Jira! This is the strong side of Jira. Besides, the integration between Jira and Confluence is extremely powerful. You can easily get your requirements from Confluence to Jira by linking a Confluence page to a Jira issue by using either Jira issue web links on Jira side or Jira issue macro on the Confluence page. Once the link between a Jira issue and a specific Confluence page is established you can easily switch between the two. You can have all the requirement details defined on a Confluence page and use the corresponding linked Jira issue to plan releases and track progress of its implementation. To keep Jira data static in Confluence to track progress, you can use the SoftComply Static Snapshots app that captures and freezes the dynamic data for you.
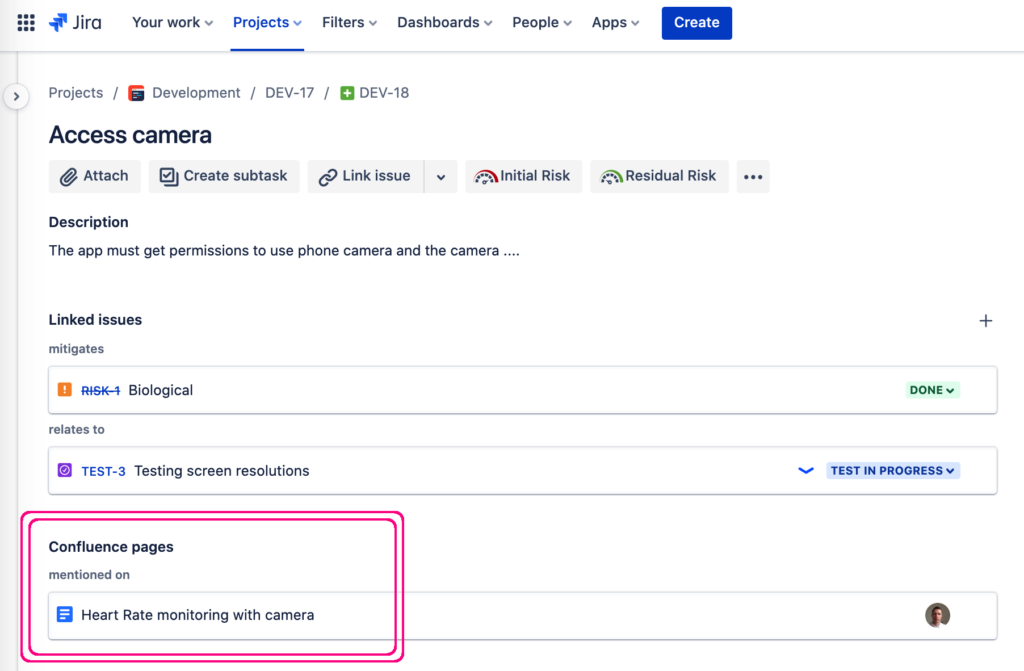
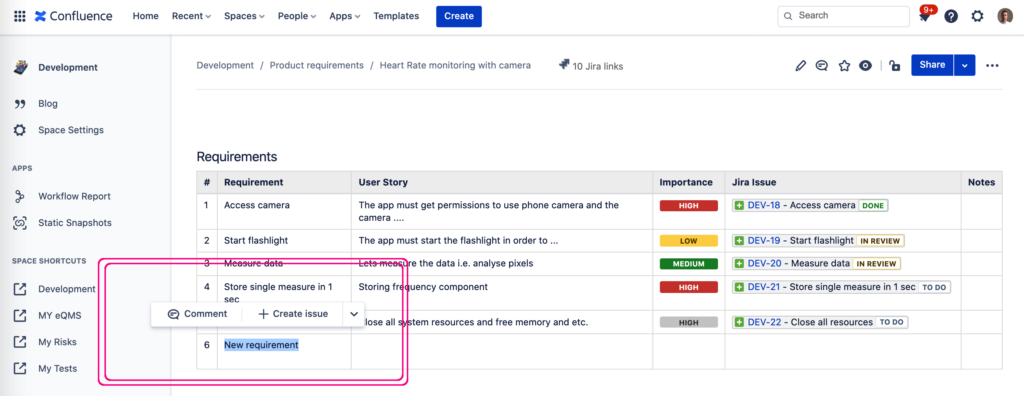
NB! Did you know that it’s incredibly easy to create a linked Jira issue straight from a Confluence page? Just select some text on the page and click “Create Jira Issue”. Or! create multiple Jira issues from the Confluence table all at once.
In case you want to have a hierarchy between the different types of requirements, you should use different Jira Software issuetypes – Epic and Story/Task. Epics represent large functional groups or main features that can be divided into more detailed features. In other words, you may want to keep the higher level user needs in Epic issues and the more detailed requirements in lower level issue types like Story or Task. That is the simplest way of having a hierarchical structure of your requirements in Confluence that can be easily carried on to Jira. In Jira, you may want to use its great reporting features and views to obtain or present a high level summary of your development status to your management.
Once you have managed your requirements, you will need to have them linked also to risks and test cases. We have built this feature in the Risk Manager apps for Jira that automatically establishes traceability between the three.
Summary
To summarise, here’s the overview of the Requirements Process in Confluence & Jira:
- Use Confluence templates to create you requirements pages in Confluence,
- Manage the requirements in Confluence during the analysis phase,
- Approve the requirements in Confluence when they are ready,
- Create placeholder Jira issues for release planning and tracking from approved Confluence requirement pages,
- Use Jira issues to implement the requirements.
Learn more
To learn more about the apps mentioned above, please see below:
- SoftComply Risk Manager & SoftComply Risk Manager Plus on Jira
- SoftComply Static Snapshots app on Confluence Cloud (try it out for free for 1 month)
- How to establish traceability between requirements, risks and test cases (video tutorials)
- How to implement eQMS Solution on Confluence Cloud
To learn more about our products for the regulated domains or to arrange a demo call, feel free to contact us.






