If you’re developing a medical device, you must prove that it is safe and effective. That proof lives in your documents: your procedures, design records, risk assessments, and test reports. Managing those documents properly is called “document control”.
At first, this might sound like an administrative detail — a few folders, some version naming, maybe a shared Google Drive. But in regulated industries, document control is the foundation of compliance. Every version, every approval and every signature tells the story of how your device came to life. If that story is messy or incomplete, your audit (and your market access) can fall apart.
That’s why so many medical device startups start out fine with tools like Google Drive until the first audit looms, or the team grows beyond two people. That’s when chaos creeps in: multiple versions of the same file, missing approvals, no clear audit trail. In this post, I’ll walk you through why this happens and how to build a compliant document management system using Confluence Cloud, the same setup that turned Anna and Mark’s startup from document chaos to audit readiness.
Watch the full walkthrough here:
See how Anna and Mark transformed their document chaos into a compliant system in under 10 minutes.
Shared Drives Work Until They Don’t
Picture this: Anna and Mark just launched their medical device software startup. They know the medical device regulations inside and out – MDR, ISO 13485, ISO 14971, IEC 62304. They even bought a ready-made eQMS package with all the document templates they need to kickstart their in-house eQMS.
Google Drive handled everything perfectly when it was just the two of them. But then they got external funding, hired three more people, and assigned specific QMS roles to each new team member.
Suddenly, nobody could find the latest version of the risk management SOP. Documents were scattered across folders with no clear naming convention. They needed separate systems for electronic signatures. And worst of all? Zero audit trail.
Sound familiar? This is where most medical device startups hit the wall.
What Makes a Document Management System Actually Compliant
A compliant document management system needs to track every single change automatically. Every document must have an owner. The approval history gets captured without manual intervention, creating a complete audit trail of who did what and when.
No files can disappear. No uncontrolled versions should exist anywhere. When an auditor shows up (and they will), you will be ready for it.
Here’s what that actually looks like in practice:
- Version control: Only one “live” version exists at any time
- Approval workflows: Documents can’t go live without proper sign-offs
- Electronic signatures: Built-in authentication for approvals
- Audit trails: Every action gets logged automatically
- Access controls: People only see what they’re supposed to see
Why Confluence Cloud Makes Sense for Medical Device Teams
Anna and Mark’s team was already using Confluence Cloud for their internal documentation. Why switch between multiple platforms when you can handle everything in one place where your product documentation already lives?
But here’s the catch – Confluence itself isn’t compliant. It lacks document versioning, approval workflows, and the traceability required for medical device regulations.
That’s where specialized apps come in. Anna and Mark discovered the SoftComply Document Manager, which transforms Confluence into a fully compliant document control system designed specifically for regulated industries.
Setting Up Your First Document Approval Workflow
Here’s exactly how Anna and Mark set up their system (and how you can too):
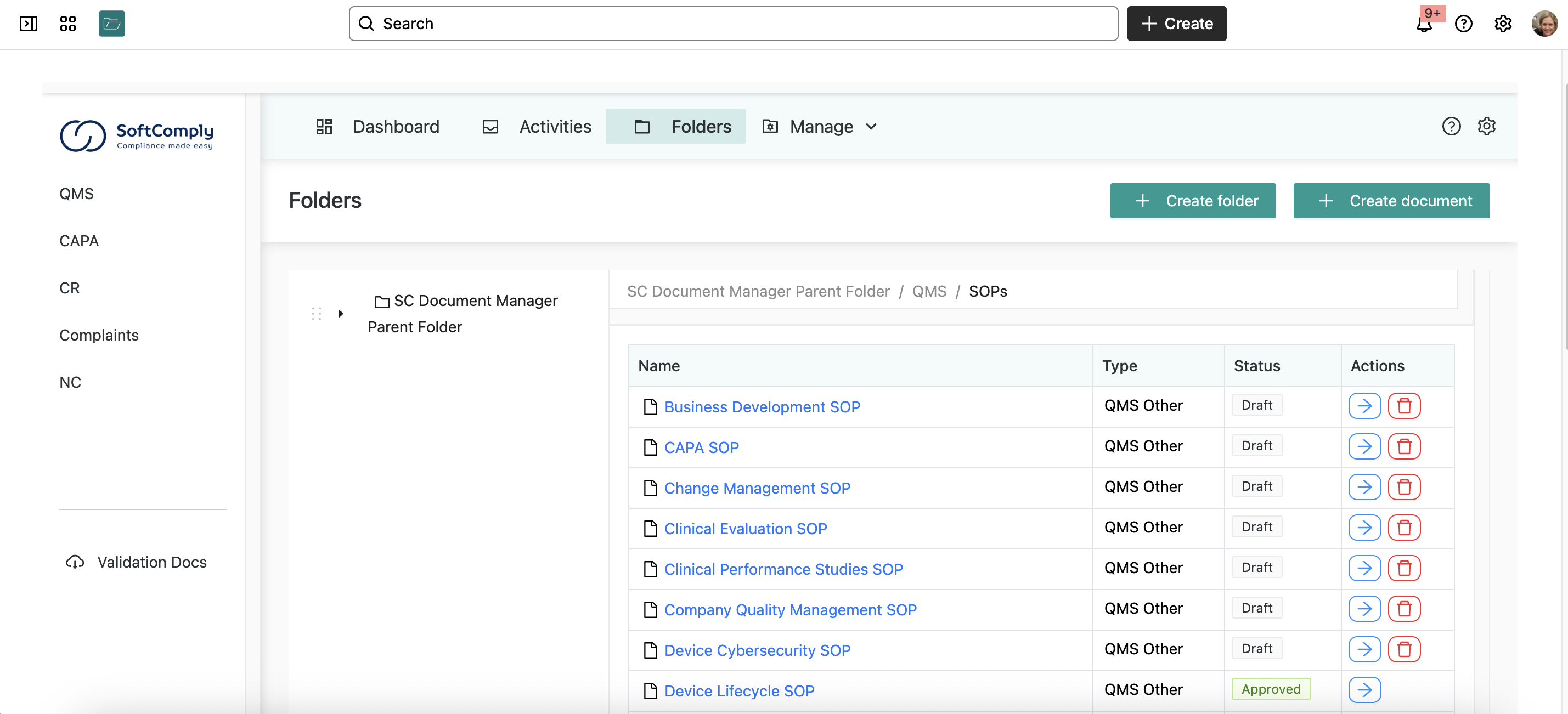
Step 1: Import Your QMS Templates
They took their purchased QMS templates and imported everything into Confluence Cloud. Using Confluence’s built-in editor, they customized each template to match their actual processes.
The key here? Don’t just copy-paste generic templates. Make them reflect how your startup actually works.
Step 2: Create Approval Activities
Instead of routing documents one by one, they created a bulk approval activity for their three first SOPs that they had drafted:
- Document Control SOP
- Device Lifecycle SOP
- Risk Management SOP
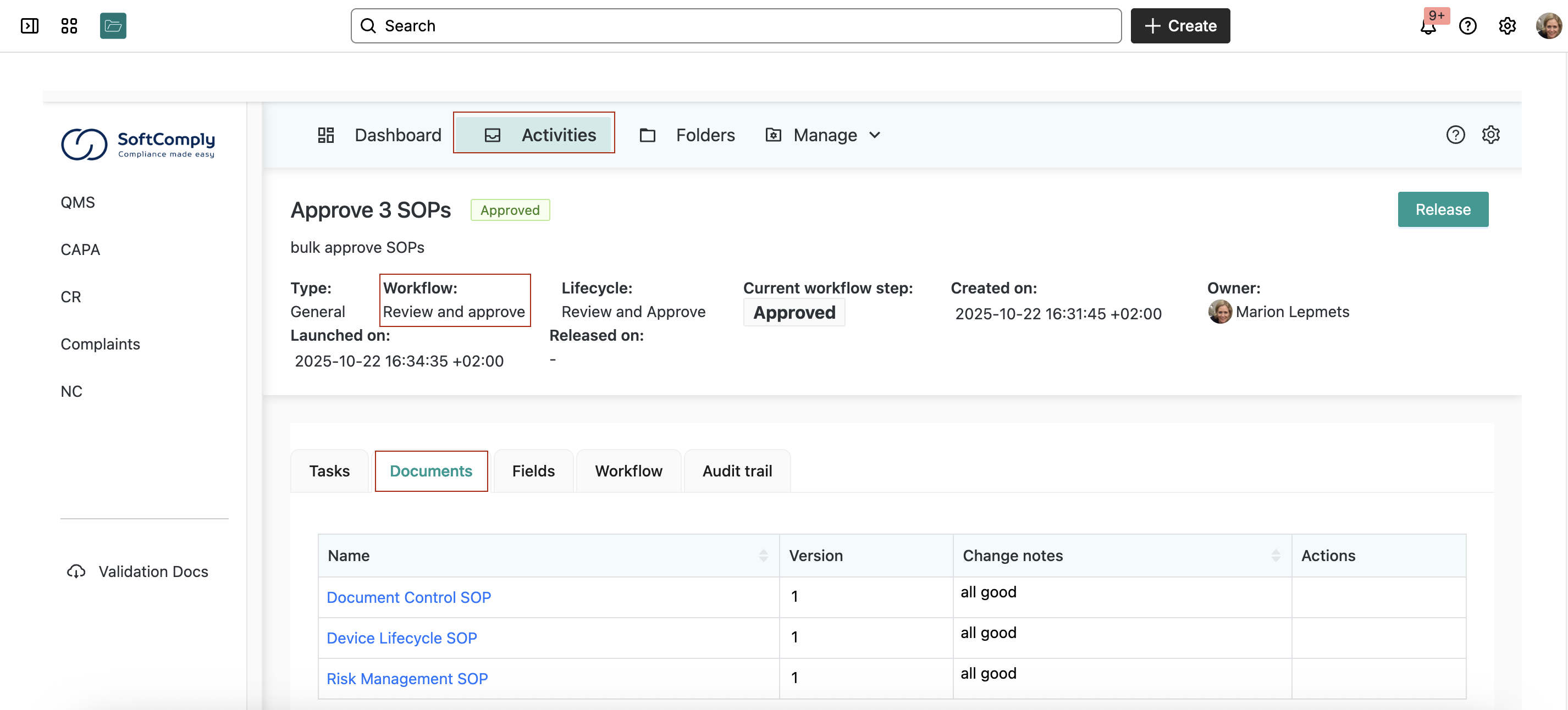
This saved hours compared to individual approvals.
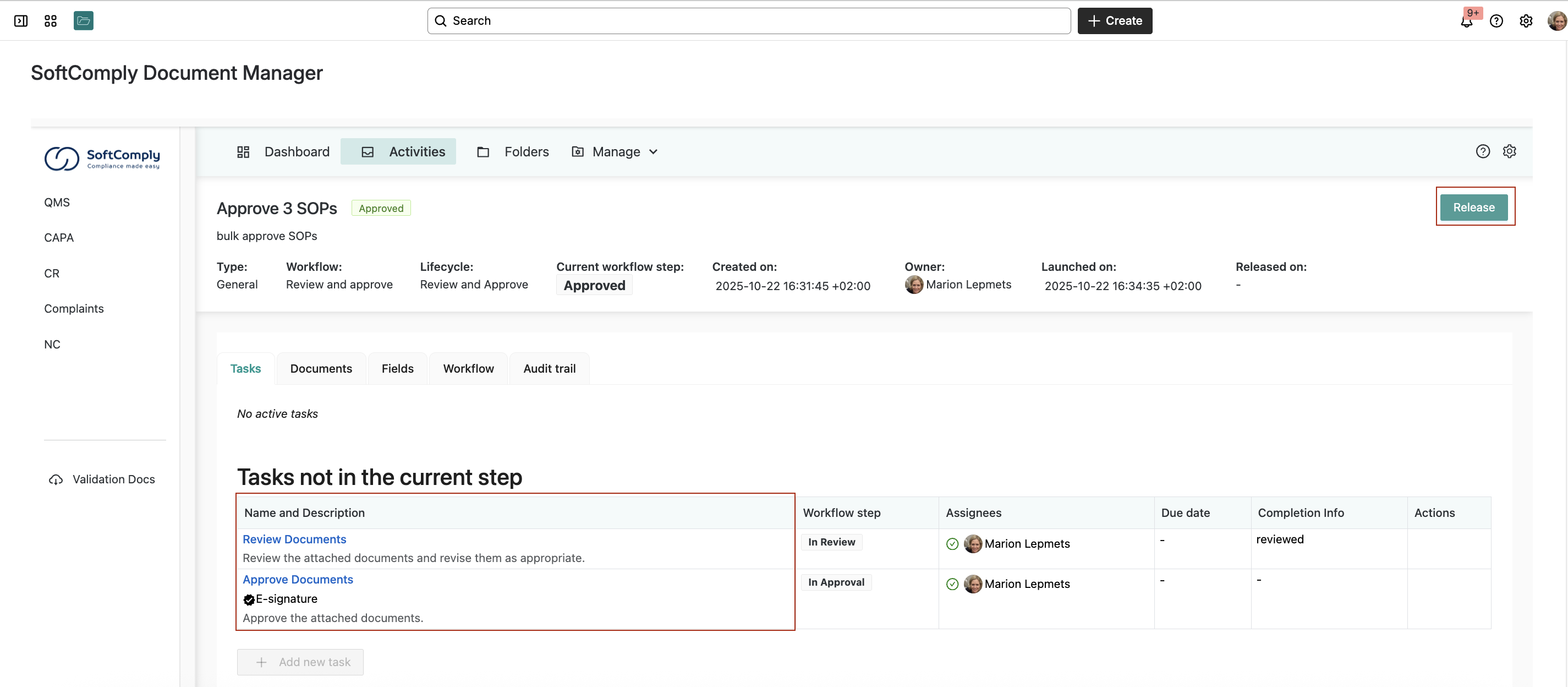
Step 3: Set Up Review and Approval Workflows
The SoftComply Document Manager comes with pre-built workflows. For their first SOPs, Anna and Mark used a simple “Review and Approve” workflow:
- Review task: Assigned team members review all the documents
- Approval task: Different team members provide final approval with e-signature
- Release: Documents become official for the entire team and locked from editing
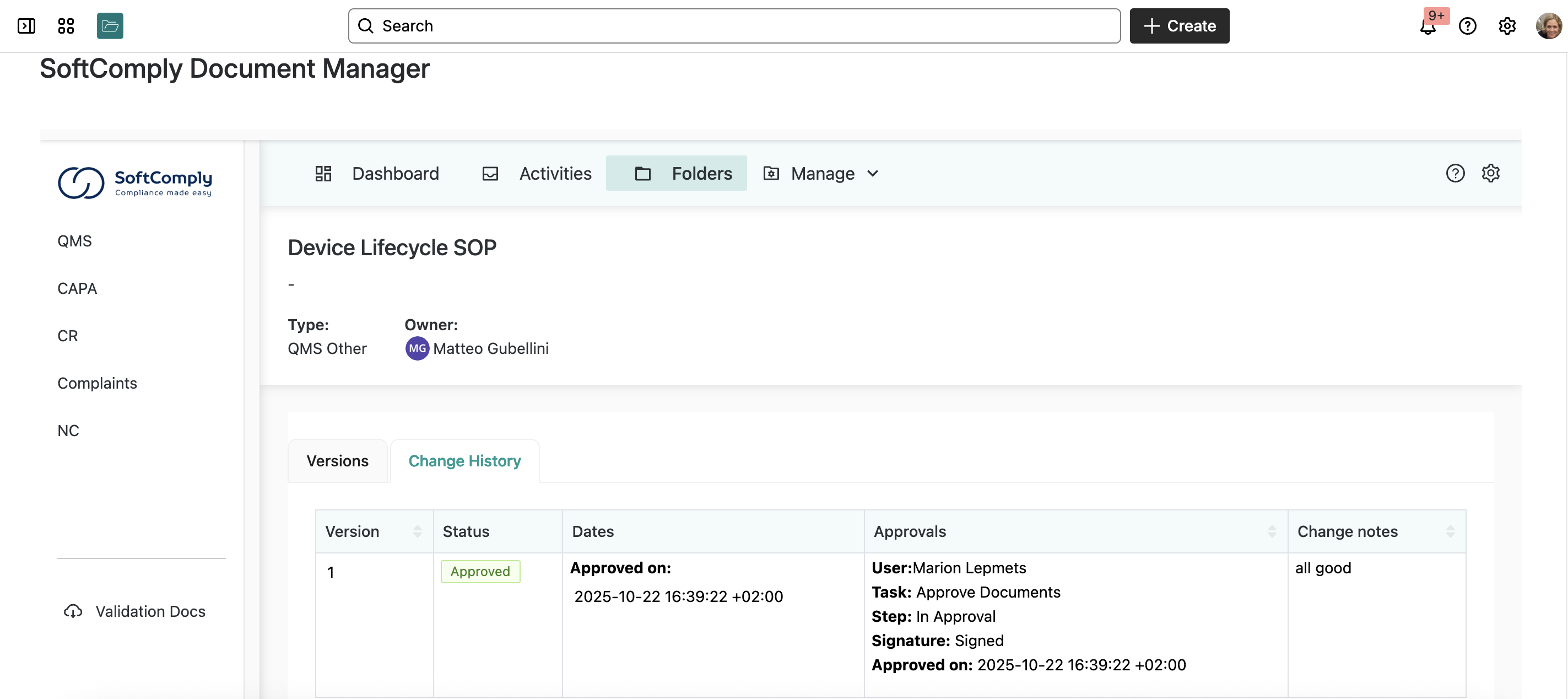
Step 4: Electronic Signatures
For compliance, approvals require electronic signatures. The system integrates with TOTP authenticators like the Google Authenticator or Microsoft Authenticator for two-factor authentication.
When someone approves a document, they enter their authenticator code. This creates a legally binding electronic signature with full audit trail.
Step 5: Document Release and Control
Once approved, documents get released to the entire organization. At this point, they become completely locked – no one can edit them without creating a new draft version.
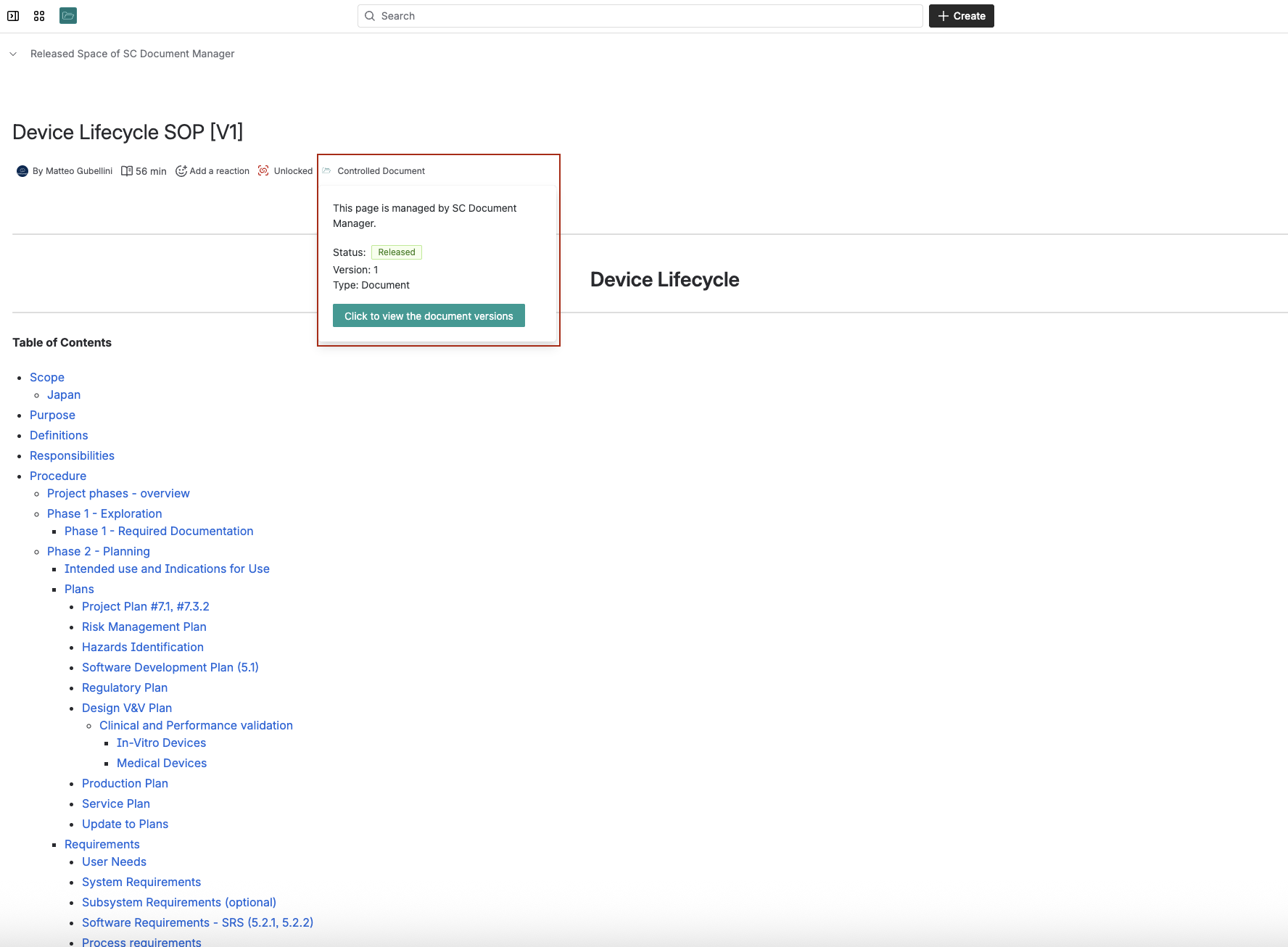
This prevents the “accidental edit” problem that plagues Google Drive systems.
The Dashboard That Actually Helps
The Document Manager includes a dedicated dashboard showing every pending task assigned to each team member.
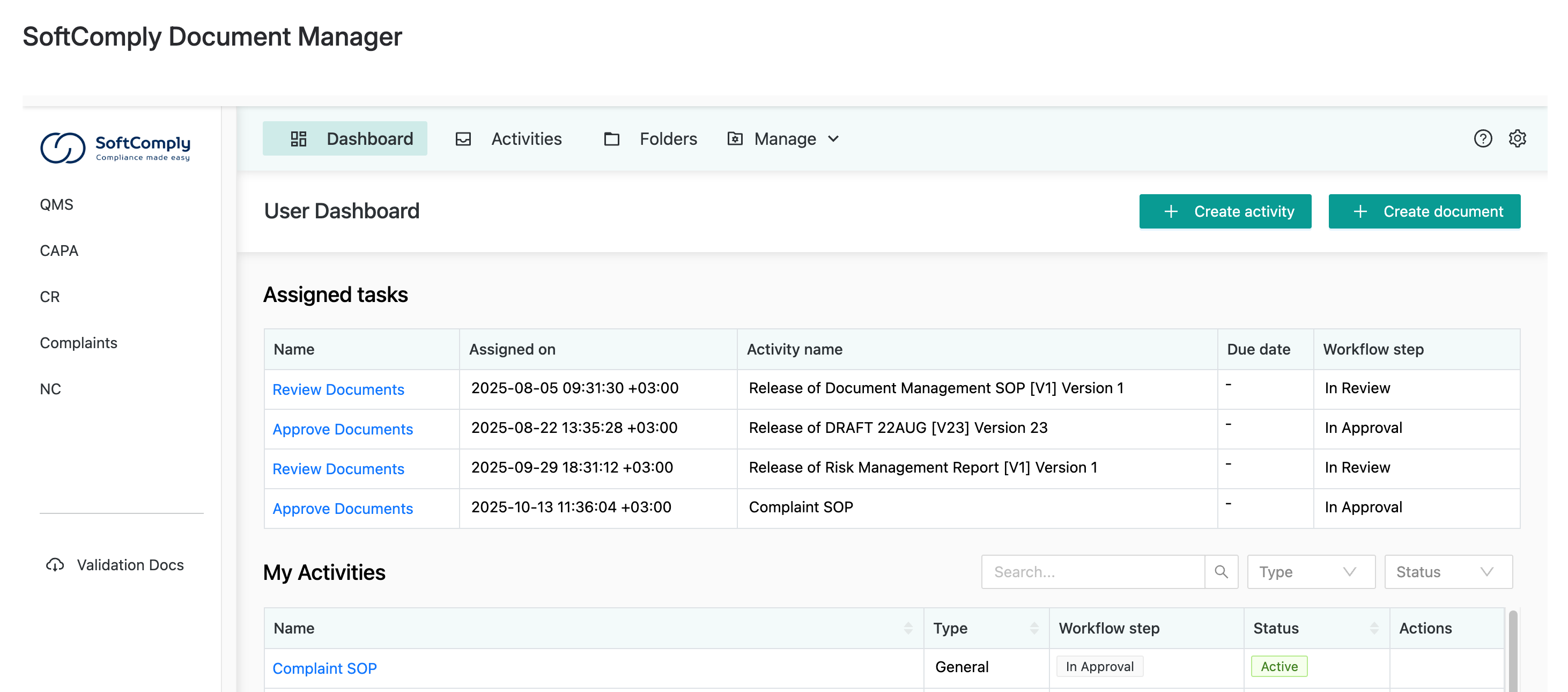
You get a familiar folder structure (so people don’t miss their old shared drive setup) plus clear visibility into what needs attention.
No more hunting through email chains or Slack messages to find out what needs your approval.
Real Benefits for Growing Teams
Here’s what changed for Anna and Mark’s team:
Before: Documents scattered across Google Drive, no version control, separate signature system, zero audit trail
After: Single source of truth in Confluence, automatic version control, built-in approvals, complete audit trail ready for any inspection
Their team can now find the latest version of any document instantly. New hires get proper access controls from day one. And when their next funding round requires compliance documentation, they’re ready.
Getting Started With Your Own System
Want to build something similar? Here are your options:
Try it out yourself: The SoftComply Document Manager offers a free 30-days trial so you can test everything with your actual documents.
Get expert help: Schedule a demo call with their team to see how the system works with your specific compliance requirements.
Learn more: Check out SoftComply’s full suite of compliance tools for medical device companies.
The bottom line? Document control doesn’t have to be painful. With the right setup, it becomes invisible infrastructure that just works – leaving your team free to focus on building great medical devices instead of wrestling with compliance paperwork.





