Traditional Risk Management process is an essential governance practice for product, portfolio, and project management. For many of us, the Risk Register is still stuck in a MS Excel spreadsheet that someone like the Project Coordinator or the Risk Manager tries to keep updated.
Here are 4 reasons that will help you get a business case approved to move your Risk Register out of Excel and into Jira:
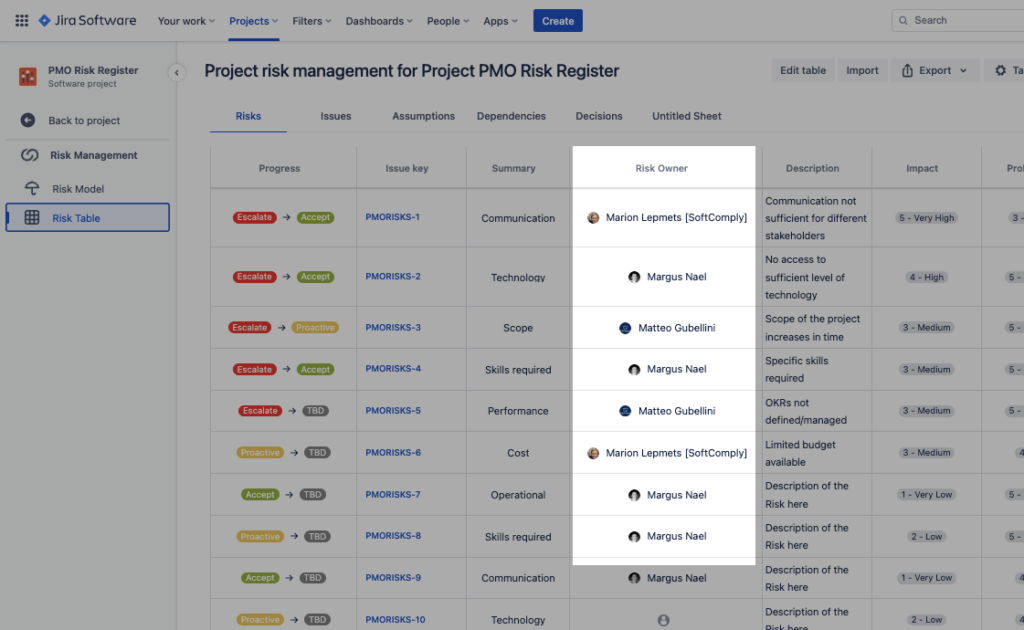
1. Assign a Risk Owner to each Risk & Automate Followups
We’ve all suffered from the barrage of email ping-pong when tracking the risk mitigation actions. Perpetually chasing nominated Risk Owners to see if something’s been done is very time consuming and can be a full time job of its own.
Having risk items in Jira allows the Project Manager to create automation rules (available out-of-the-box) for sequenced follow-up of outstanding tasks to a due date.
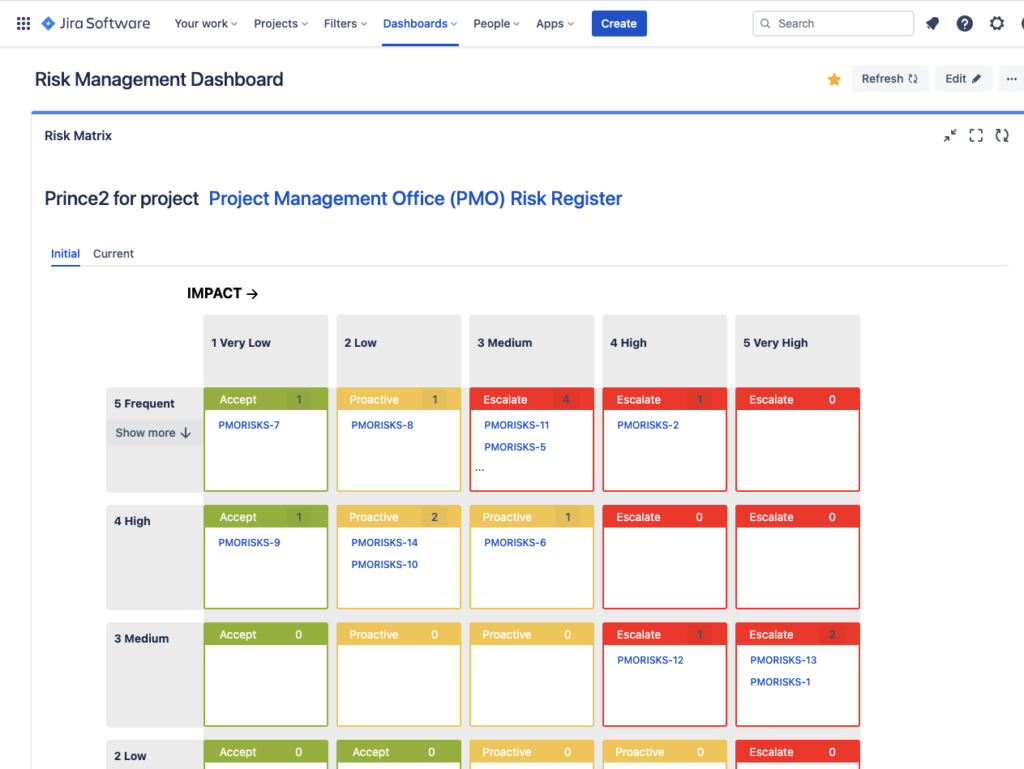
2. Instant & Always Up-To-Date Risk Matrix Views in Jira Dashboards and Confluence
While Excel has good features for various diagrams (including those pivot tables that seem to be constantly breaking), the SoftComply Risk Manager apps for Jira have a very cool risk matrix gadget that can be added to a Jira reporting dashboard in seconds.
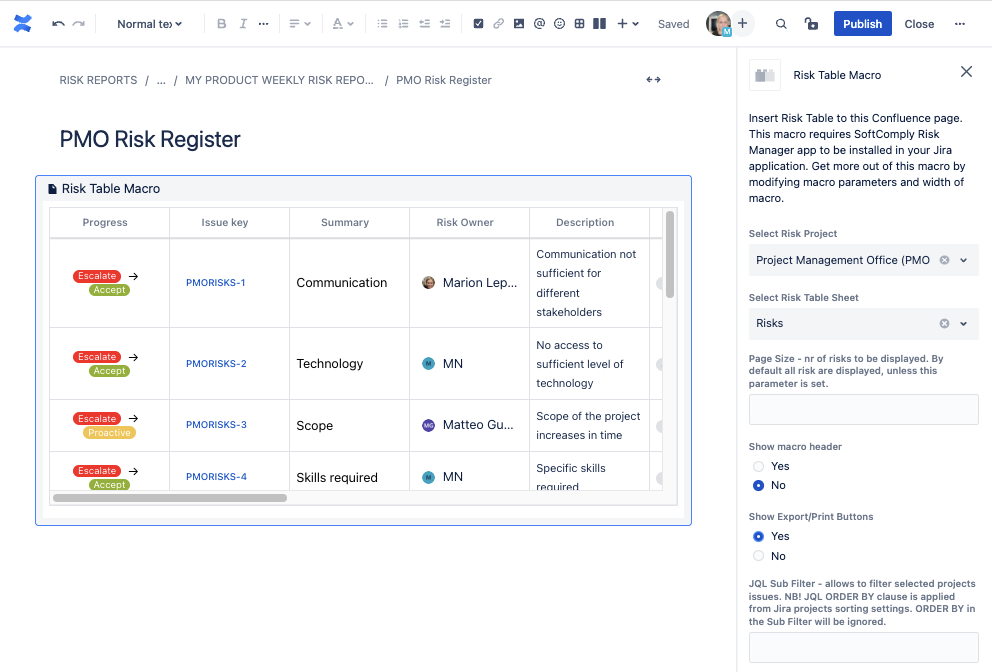
Furthermore, you can add the SoftComply Risk Reports to any of your team’s Confluence page. These reports are automatically updated in real time.
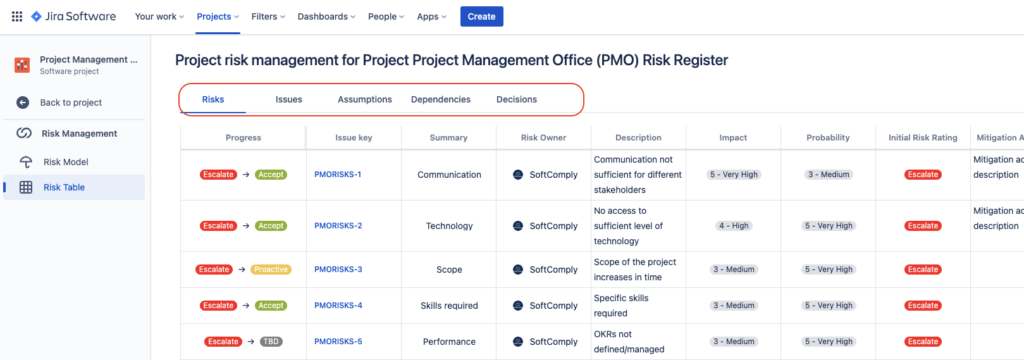
3. Empowering Teams to Manage Risks Themselves
Let’s face it, a Risk Register in an Excel file buried four folders deep within your project governance doesn’t make it easy to find or know which file version is the latest to update. By empowering delivery teams to raise and mitigate their own Risks, Issues, Assumptions and Dependencies in the same Jira Project that they use to manage delivery every day, you get the potential to have a more proactive risk management culture.
You can keep different types of risks in different Sheets of the Risk Table for better risk management and overview:
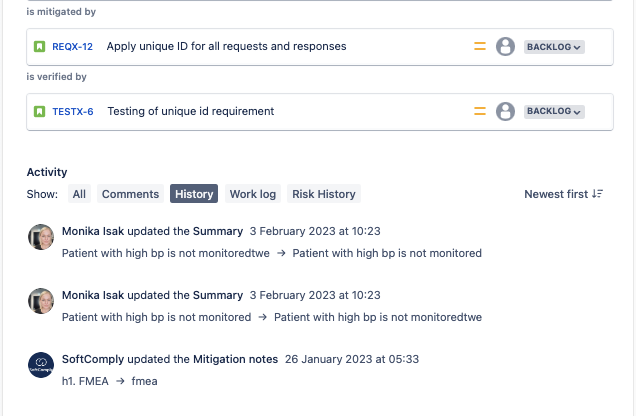
4. Governance of Audit Trail of Changes
A Risk Register in Jira gives the ability to see the full history of who created, edited and worked on every risk item – it will make the Risk & Compliance department your best supporter. This is even more critical for the Regulated Industries of Banking and Finance or the Medical Devices.
Want to Learn More about the Risk Manager apps on Jira Cloud?
Download a free 30 day trial of the SoftComply Risk Manager apps in the Atlassian Marketplace. They are available for both Jira Cloud and Data Centre.
- For one 2-dimensional risk matrix per Jira project: SoftComply Risk Manager for Product Risk Management
- For multiple more advanced risk matrices to be shared across many Jira projects: SoftComply Risk Manager Plus – for Safety-Critical Products
Get a free consultation to quickly discover which SoftComply apps would best fit to your organisation: Click here to book an appointment with Sales:
SoftComply partners are passionate about helping Project Managers, PMO Managers and Risk Managers get their Risk Registers into Jira by providing a managed service to:
a) setup your chosen SoftComply Risk Manager app for Jira; and
b) Write the technical specifications to cover your existing Risk Manager excel spreadsheet; and
c) Configure the SoftComply Risk Manager app for Jira to your requirements including setting up reporting with the Risk Matrix gadget installed on a Jira Dashboard.
Click here to book a free Managed Services appointment so we can get you a quote.





