Paper-based Quality Management Systems are now (almost) a thing of the past (it’s sooo 20th century…).
But in some cases, printing records and documents from the eDMS is necessary, or at least exporting them to PDF. The requirements for paper documents are the same as digital documents. But, unlike electronic formats, physical misplacement or loss of pages is a real risk. The solution is quite simple: page numbers. This will allow the user to easily sort the pages and determine what the last one is.
Each page should have its number in the following format – “Page X of Y”.
It seems obvious, but check how many times you have received documents from a bank or a lawyer without page numbers.
In Atlassian Confluence, pages are a continuous stream of content and there is no paging as such. The built-in PDF export does not add page numbers by default. Some apps are available to heavily customize PDF layouts, but for a simple page numbering we can provide you with a quick and easy solution using CSS for PDF Stylesheets.
A simple 3-step guide to adding page numbers to your Confluence page PDF exports:
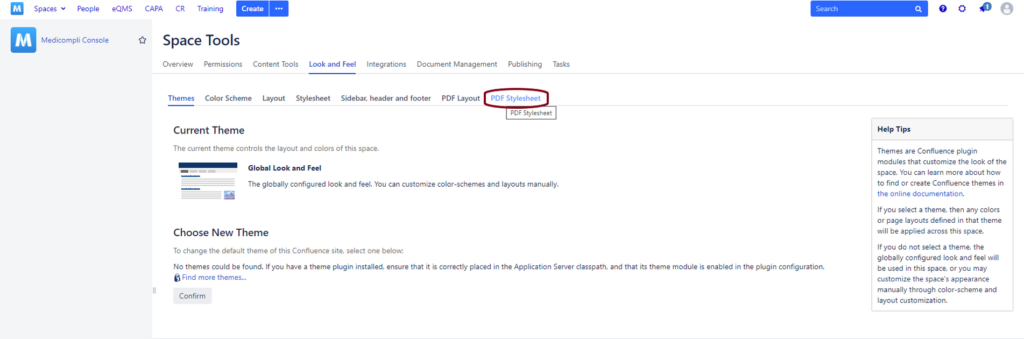
1. First, go to the “Space Tools” in the Confluence Space in which you want to add page numbers for PDF exports, and select “Look and Feel” from there.
2. From the Look and Feel page, select the PDF Stylesheet
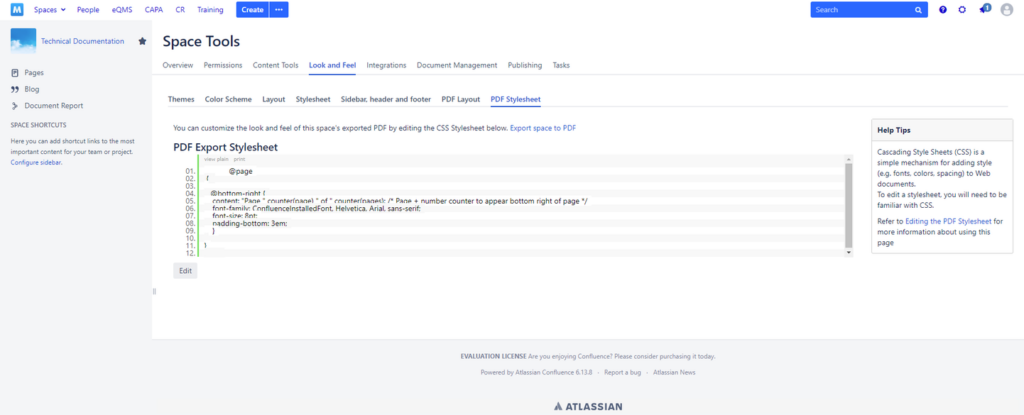
You are now on the PDF Export Stylesheet page that you can edit further and add the page numbering in CSS
3. Paste the code below into the PDF Export Stylesheet to get the page numbers in your PDF Exports in the format of PAGE X of Y
@page
{
@bottom-right {
content: "Page " counter(page) " of " counter(pages); /* Page + number counter to appear bottom right of page */
font-family: ConfluenceInstalledFont, Helvetica, Arial, sans-serif;
font-size: 8pt;
padding-bottom: 3em;
}
}
You will now have page numbers in the format of Page X of Y on each PDF Export that you’ll make out of any page in this space!