Picture this: You have just finished writing your requirements specification and saved it as “Requirements_final.doc”
Then come the edits, code reviews, and compliance feedback.
Suddenly you’re looking at three files:
- “Requirements_final_v2.doc”,
- “Requirements_final_really_final.doc”, and
- “Requirements_fixed_final.doc”.
Which one’s actually the final one? You send one to the team, but they build from another. Suddenly, the project’s out of sync. Deadlines slip. Rework piles up.
For most teams, this is just frustrating. But in regulated industries like medical devices, pharma or automotive? It’s downright dangerous.
Watch my video breakdown of document control challenges and solutions:
Let’s say a software company is building a medical device app. The design team updates a specification file to include a critical safety alert, but manufacturing receives an older version. The alert never makes it into the final product. The device ships. And the defect appears in the field.
At that point, it’s not just a versioning mistake anymore – it’s a regulatory nightmare.
Studies show that missing or incorrect documentation is the most common root cause for Class I device recalls. That’s why regulators expect teams to control every document, every requirement, every test with full traceability.
Why Confluence Alone Isn’t Enough for Document Control
Confluence is fantastic for online collaboration. It’s got a simple editor and basic permission management. But here’s what it’s missing for regulated industries:
- No proper workflows – Everything’s manual
- No electronic signatures – Can’t meet compliance requirements
- Basic versioning – Stacks versions on top of each other, always showing latest content
- Manual document locking – No automated controls
Confluence works great for company intranets and information sharing. But it doesn’t tick all the boxes needed for solid document management in regulated industries.
How SoftComply Document Manager Fills the Compliance Gaps
That’s exactly why we built SoftComply Document Manager. It’s designed to integrate perfectly with Confluence Cloud while adding all the compliance features you need for document control.
Out-of-the-Box Compliance Features
The Document Manager app is MDR, IVDR, and 21 CFR Part 11 compliant document management system with electronic signatures. You get configurable document approval workflows that cover the entire document lifecycle.
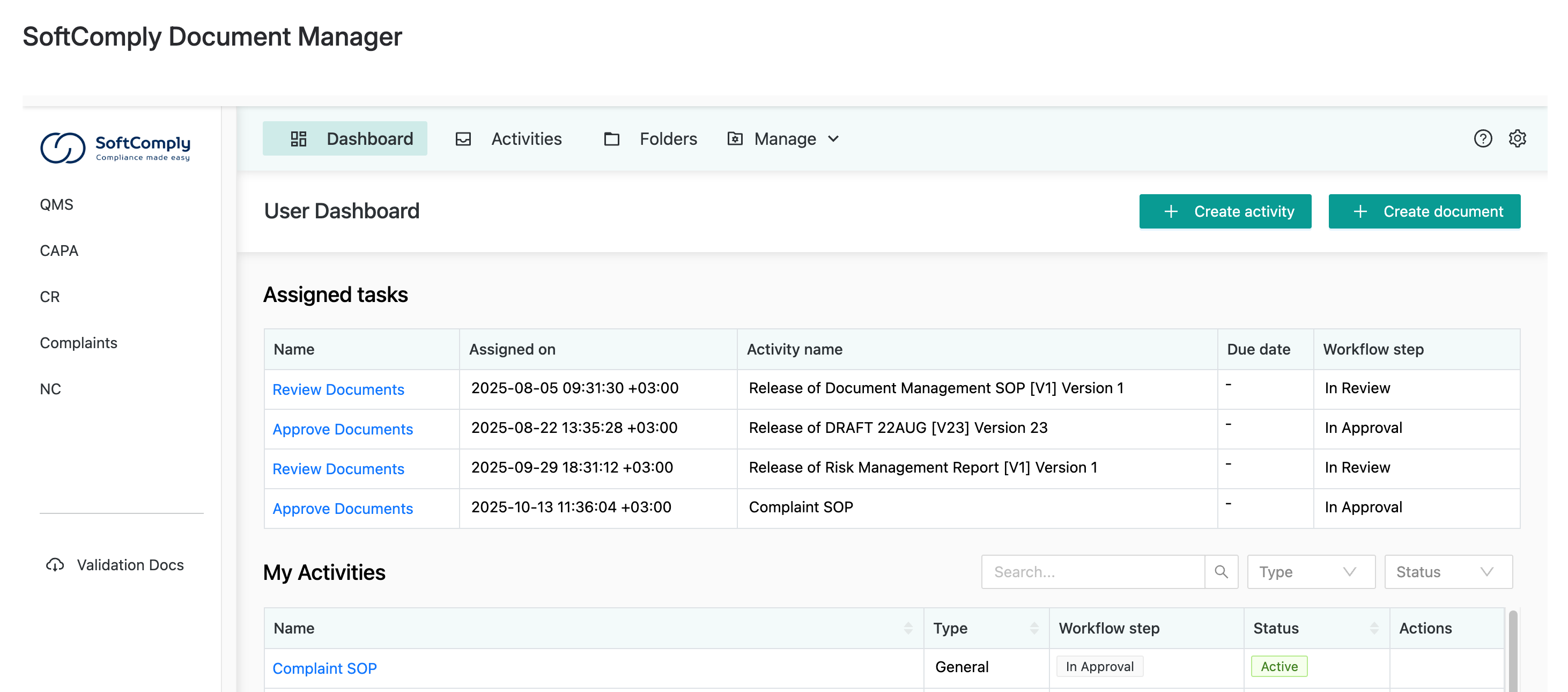
Dedicated Task Management
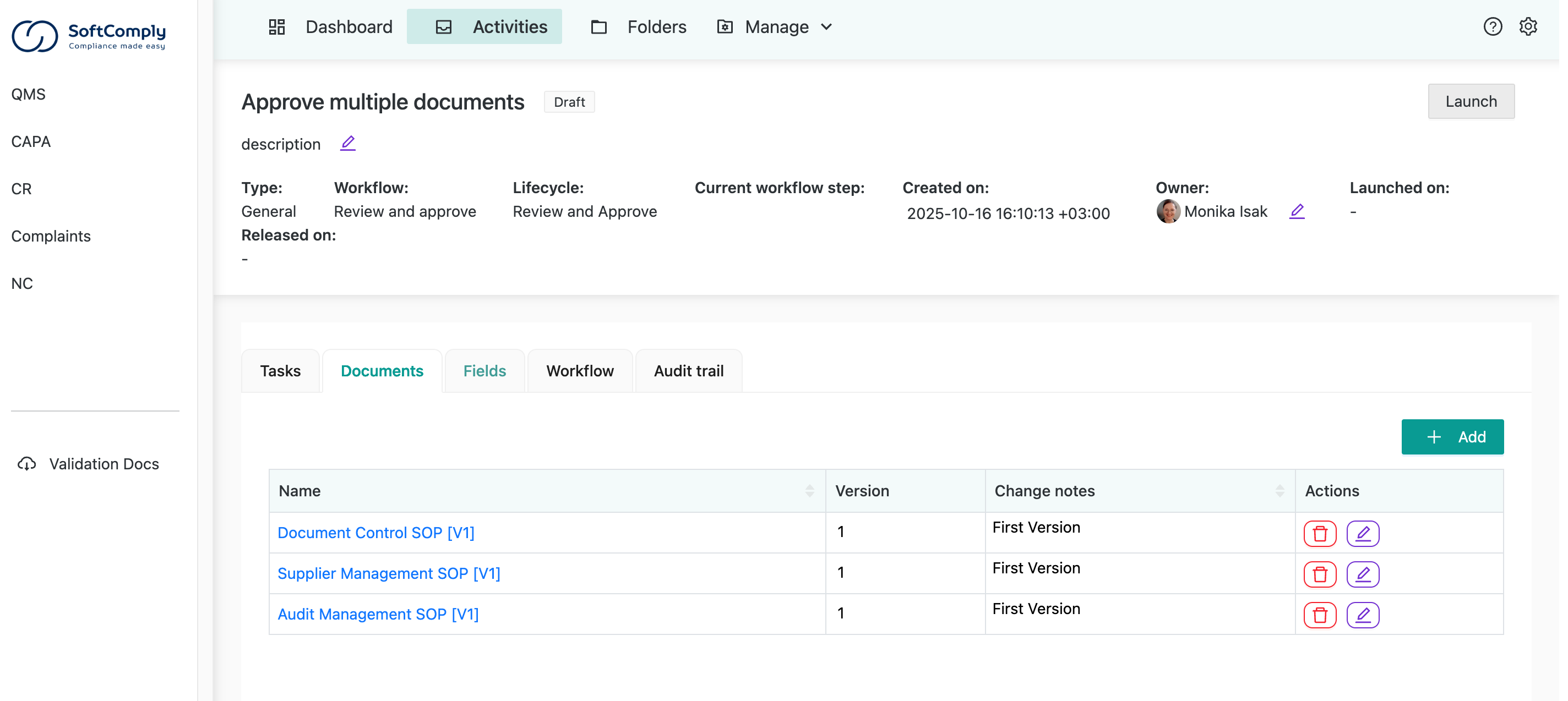
There’s a dedicated dashboard for all your document approval tasks. You can easily track all document management activities assigned to you, approve multiple documents simultaneously, and view full document version history on each Confluence page.
Compliance Modules
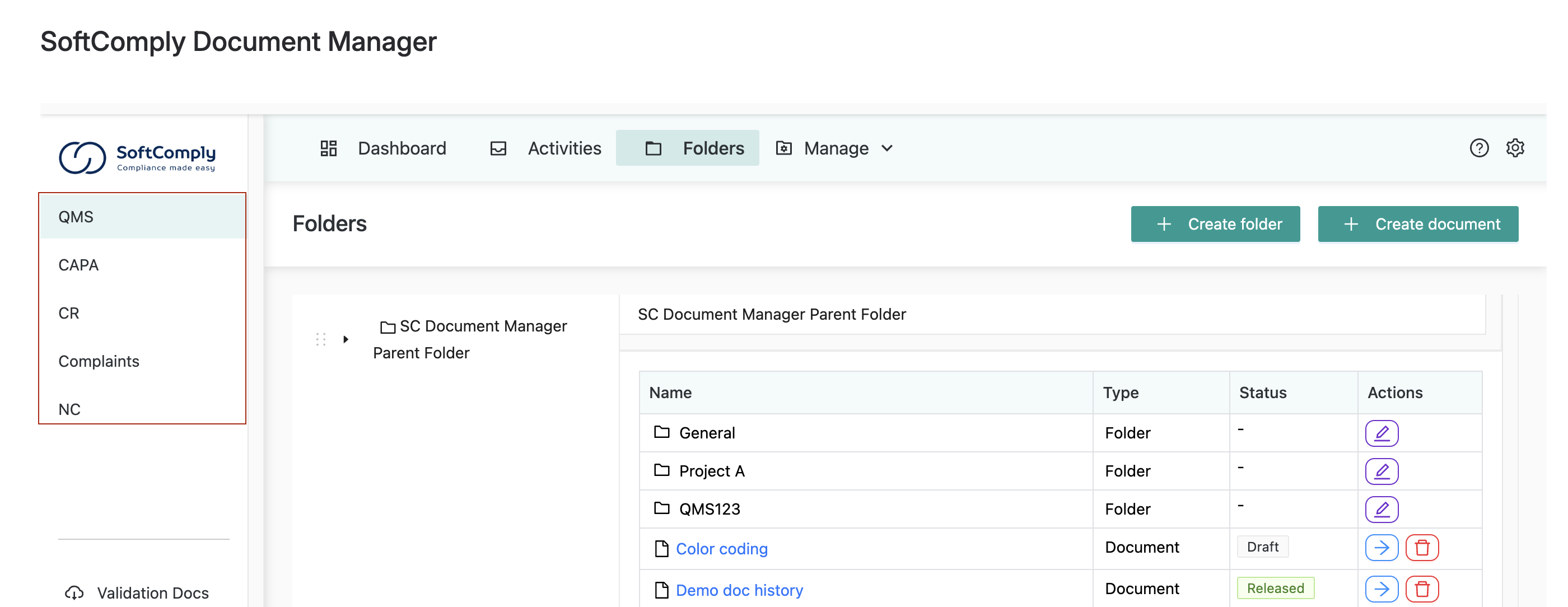
SoftComply Document Manager is the only document management app on Atlassian Confluence Cloud with all necessary modules for compliant document management system, includes modules for:
- CAPA (Corrective and Preventive Actions)
- Complaints management
- Nonconformities tracking
- Change requests
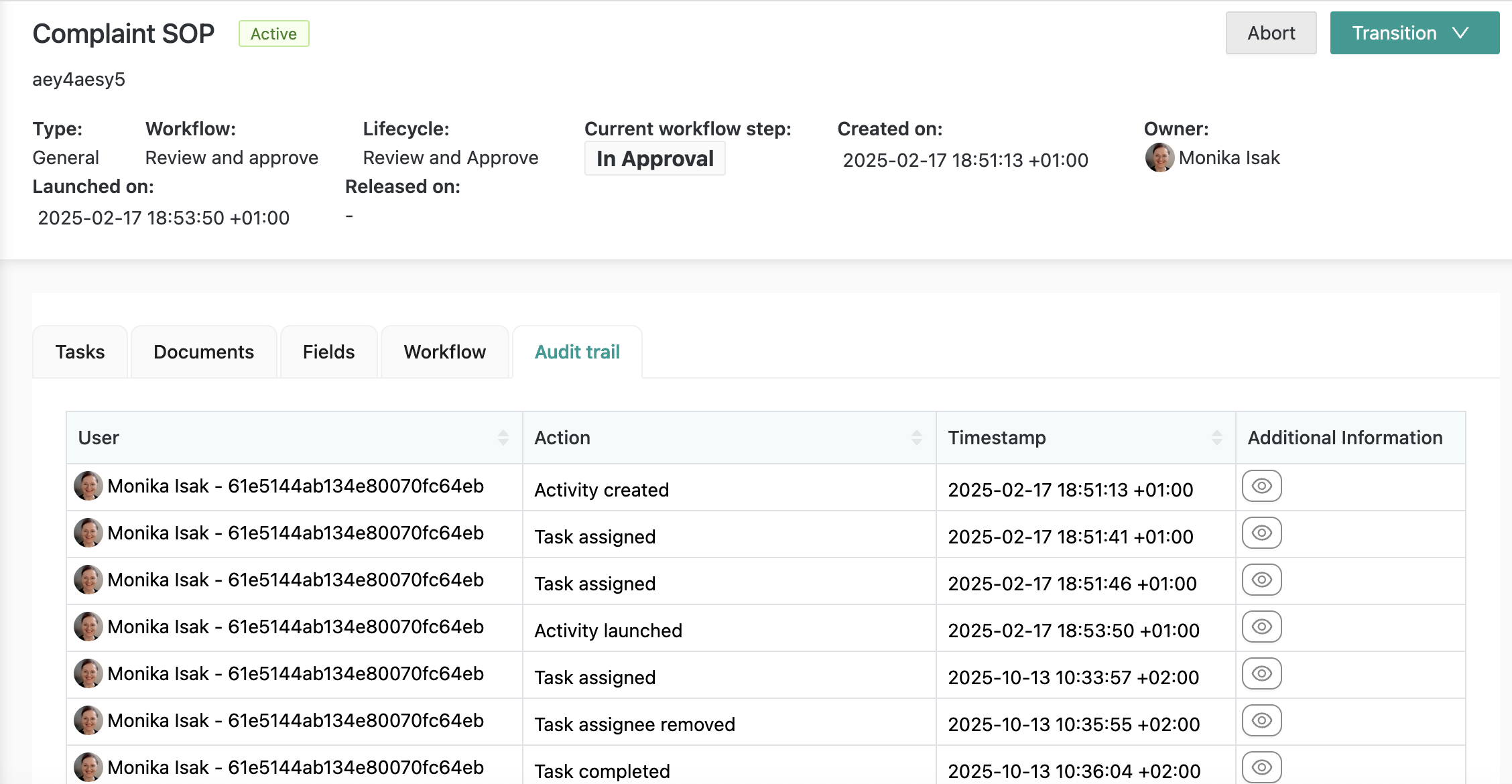
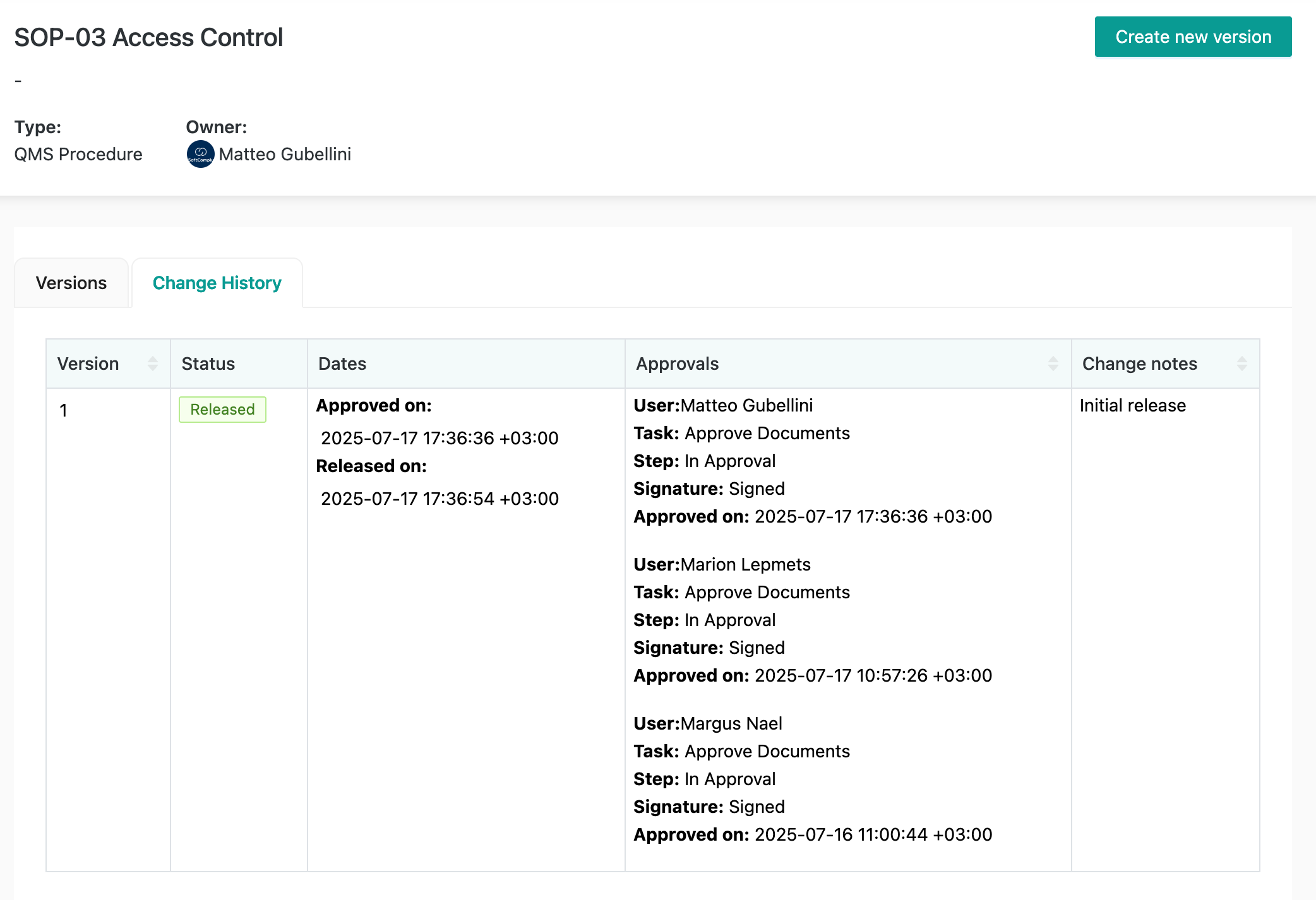
The Document Manager app provides a full audit trail with “who did what when” details:
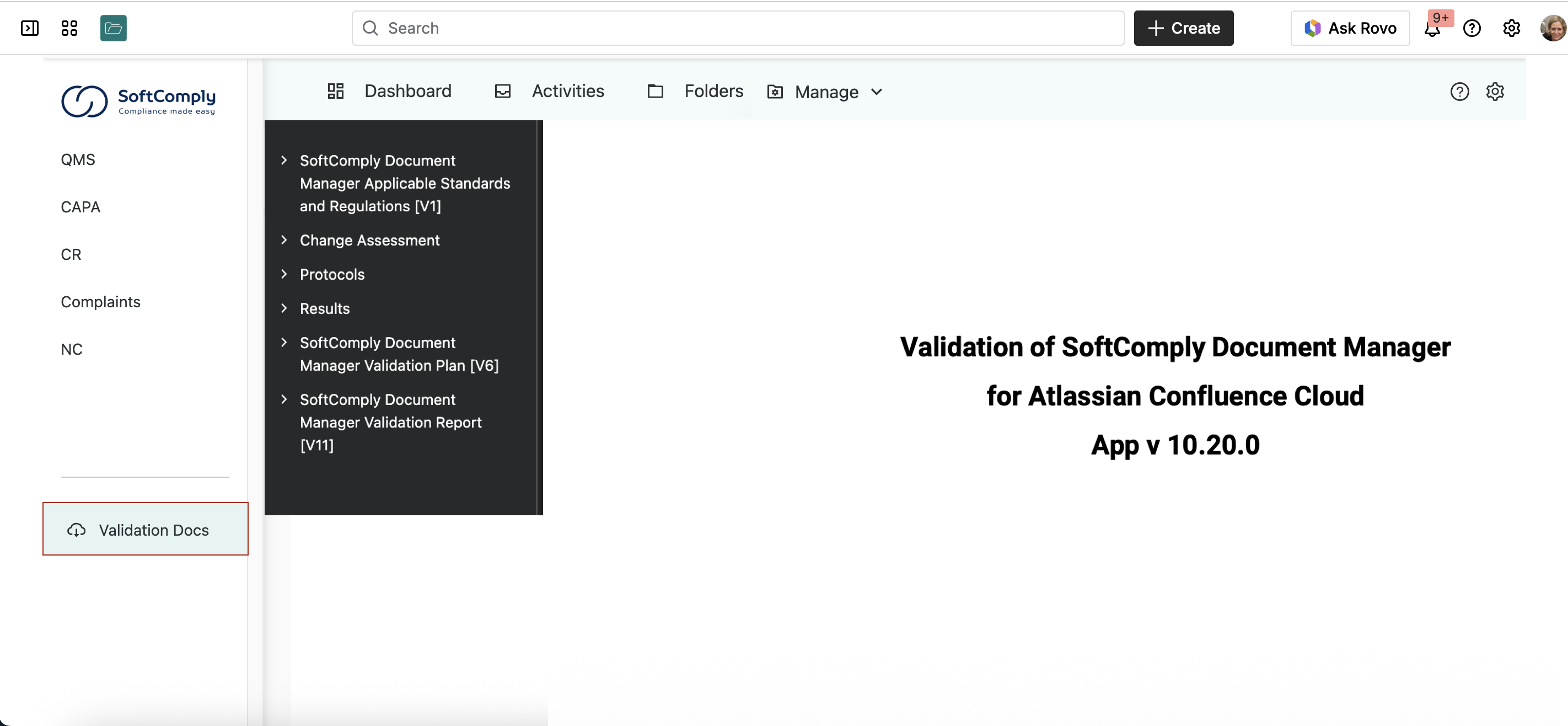
It’s also the only Confluence-based document management system that’s validated:
How Document Approval and Versioning Actually Works
SoftComply Document Manager is built on Confluence Cloud so you can edit documents in Confluence and use all the powerful Confluence macros, plus the ones that the Document Manager app adds.
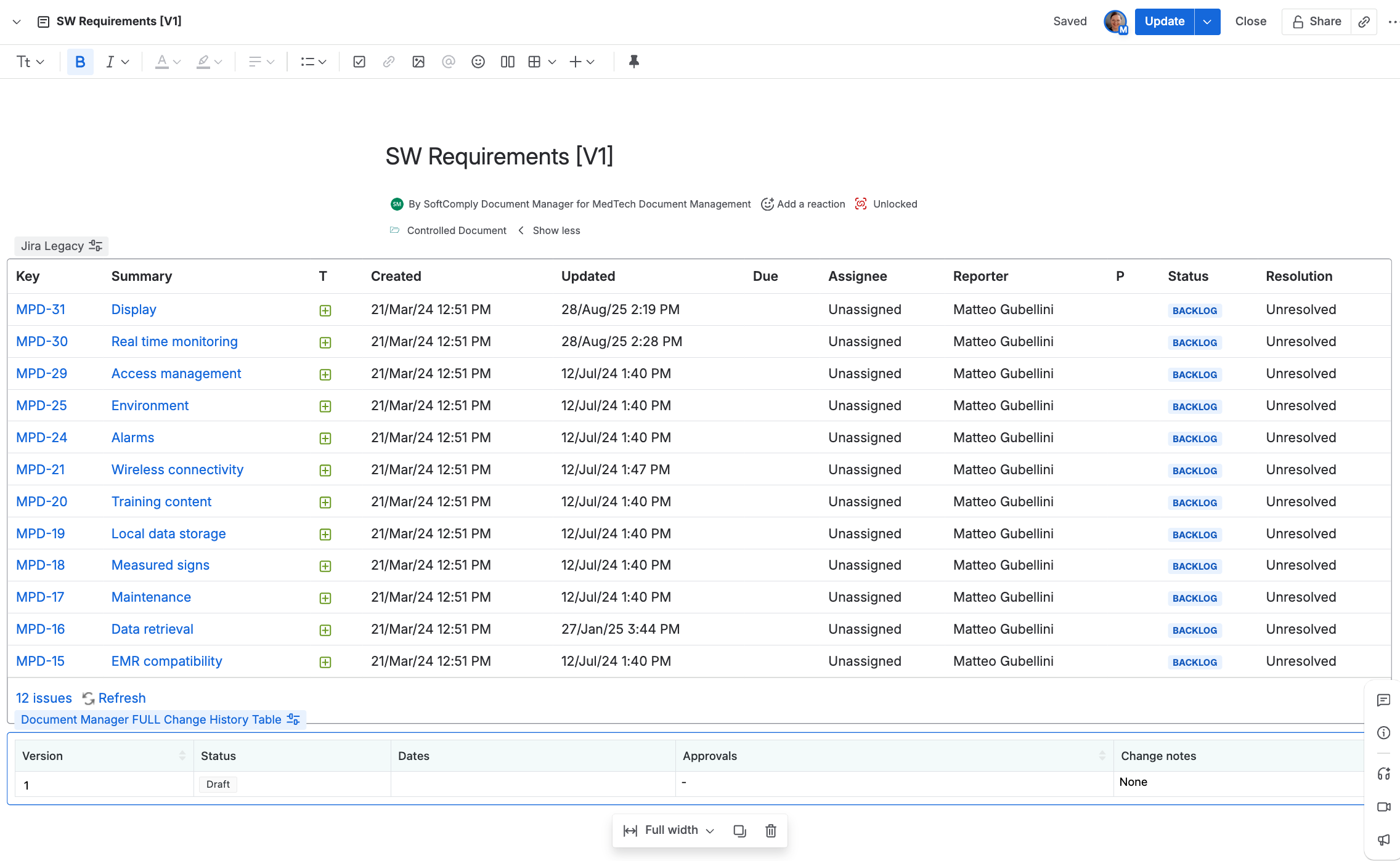
Document navigation uses a familiar folder structure.
Each document version becomes a separate page in Confluence. And each document has an automated change history table on it with all the necessary details about its approval history:
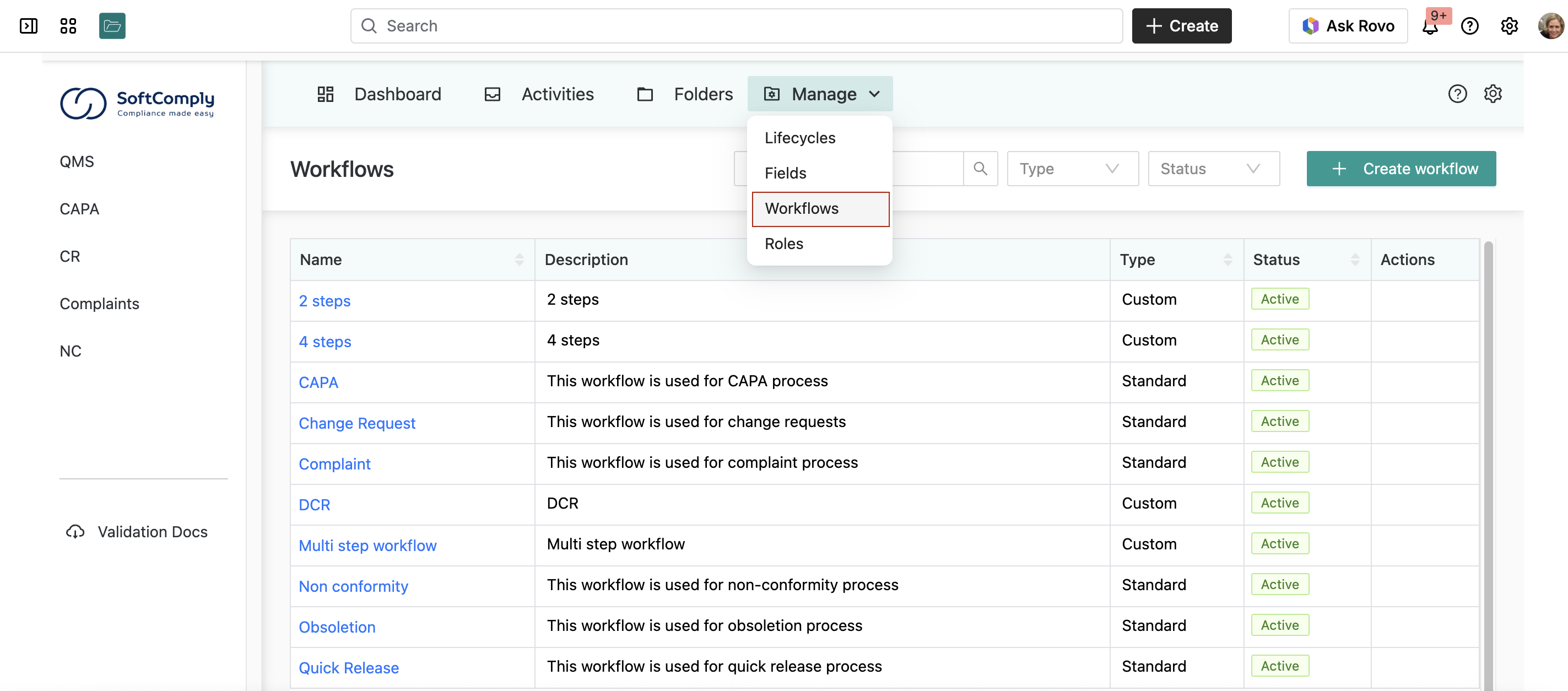
Documents can be approved using out-of-the-box workflows, or you can build custom workflows for your specific needs:
In SoftComply Document Manager, you can approve multiple documents at once with a single signature:
Users get assigned to specific workflow tasks, and you can check your task list in the app dashboard:
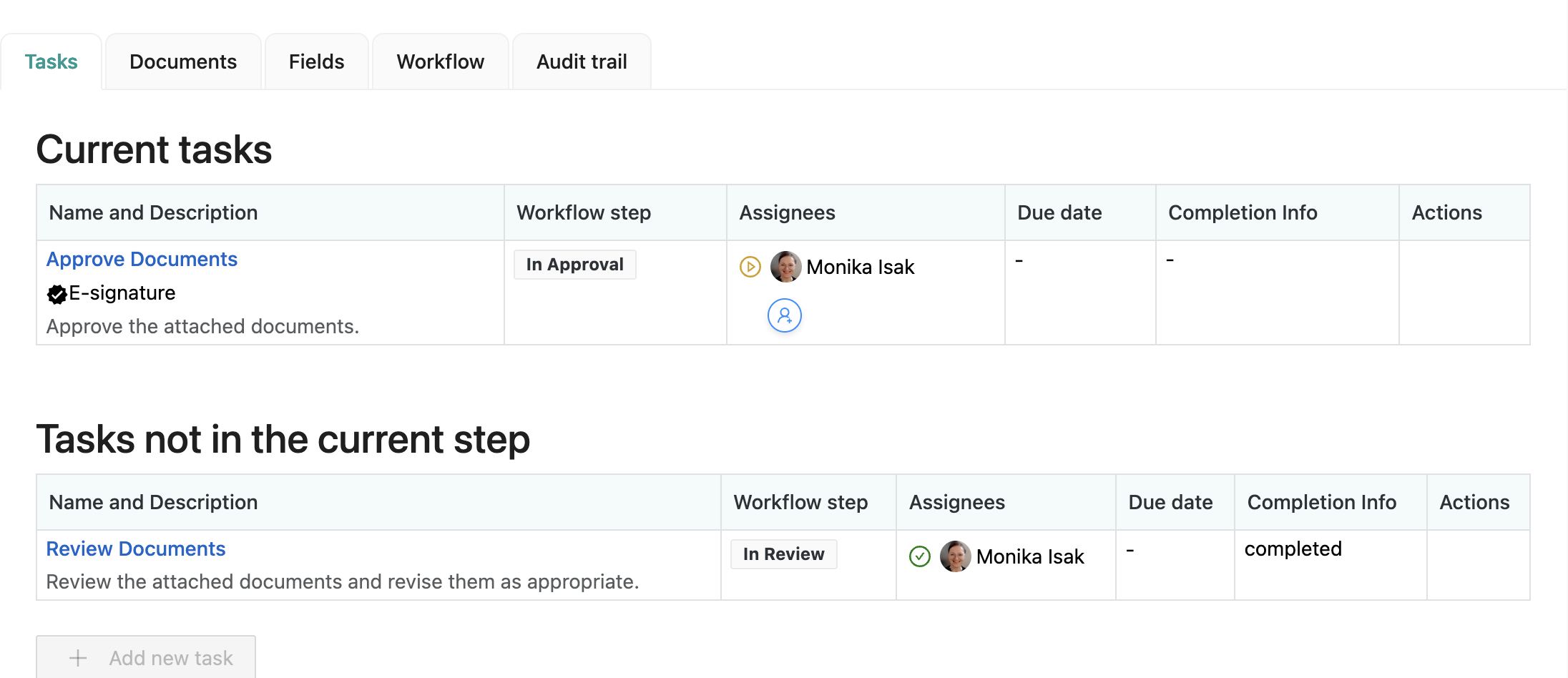
You can also check your document management tasks on your Confluence homepage:
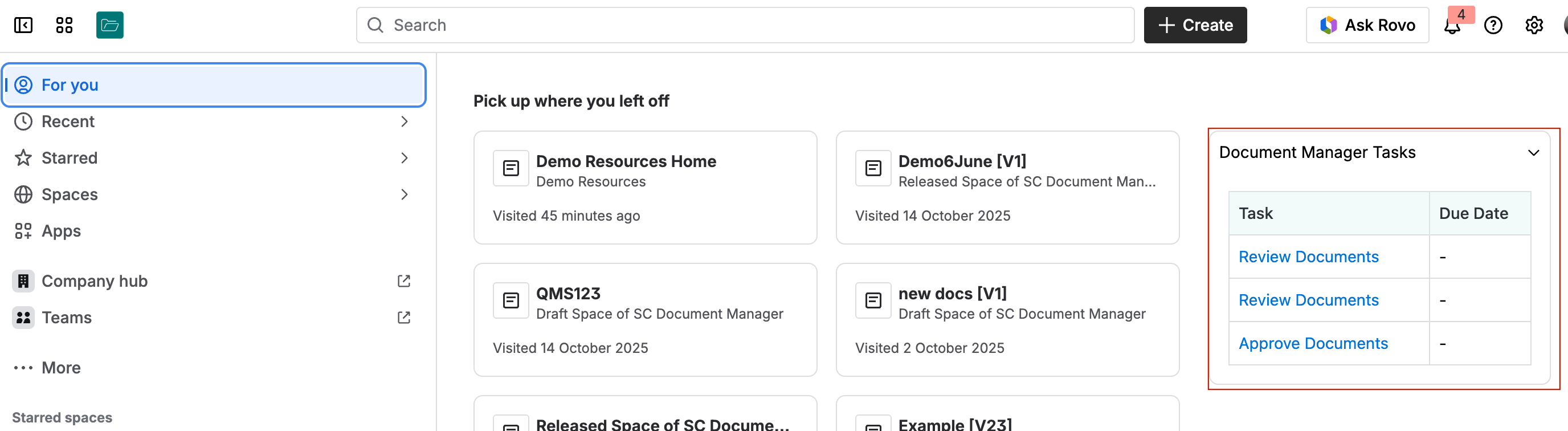
Compliant Electronic Signatures in Confluence
Tasks range from simple actions (like reviewing a document) to legally binding electronic signatures. The Document Manager app automatically locks documents when they shouldn’t be editable anymore.
Signatures use standard TOTP authenticators like Google Authenticator or Microsoft Authenticator.
Smart Status Management
In the “approved” status, a document has all required signatures but isn’t public yet. This is perfect for scenarios where a procedure has been approved but is waiting for training before release (which happens with a single button click).
When you need to create a new document version, a simple interface lets you do that. The app automatically manages previous versions, making them obsolete when a new version is published.
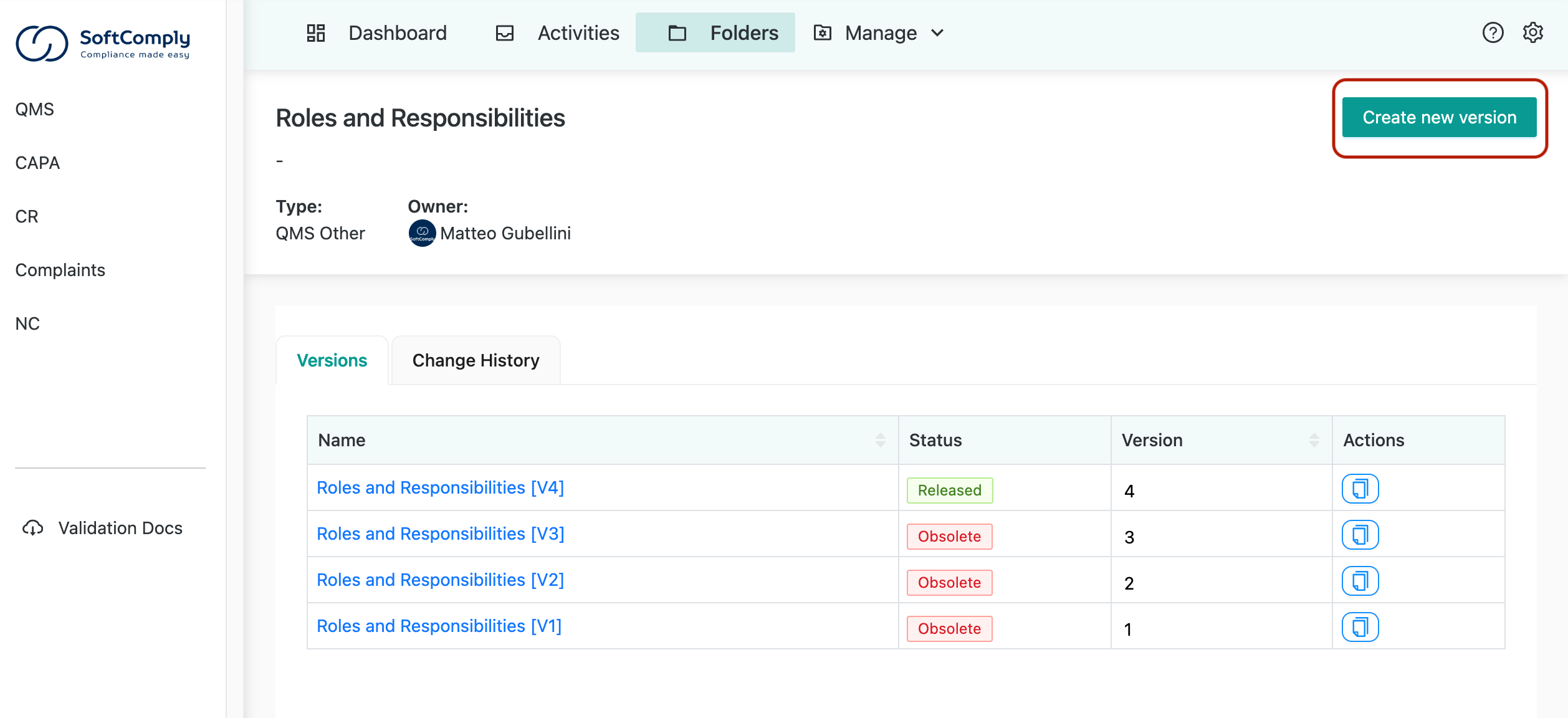
Never Guess Which Version Is “Really Final” Again
For regulated industries, this isn’t just about efficiency – it’s about safety. With SoftComply Document Manager, you’ll never have to guess which version is the real final.
The system maintains full traceability between requirements, tests, and outputs while keeping you audit-ready without the chaos of manual versioning.
Ready to eliminate document version chaos in your regulated environment?
Try SoftComply Document Manager free on the Atlassian Marketplace, or schedule a demo to see how it works with your specific use case.
For teams already managing complex risk scenarios, you might also want to check out SoftComply Risk Manager Plus – the most advanced risk management app on Jira Cloud. It supports ISO 14971, ISO 27001, FDA, and NIST frameworks while integrating seamlessly with your existing Atlassian tools.
Teams using SoftComply Risk Manager have increased efficiency and reduced costs by a factor of ten compared to Excel-based processes. If you’re developing Class 2/IIb medical devices or working in other regulated industries where compliance isn’t optional, it might be exactly what you need to get innovative products to market faster.





