Your Development team might find the instructions from Quality and Compliance team time-consuming – slowing down their delivery time, but the regulatory standards are put in place to ensure the safety, effectiveness and integrity of products from different industries. There are significant consequences for non-compliance ranging from loss of business and legal penalties to your products being recalled from the market, depending on the compliance requirements of your industry.
Let’s Break the Silos
Your Quality team knows the regulatory requirements that your organisation and products need to meet. They make sure that the entire company follows these in their everyday work. Most other teams do not have fully appreciate the regulatory requirements and simply follow their process description and instructions. But these small steps help the Quality team measure the overall progress towards compliance in the organisation.
Being compliant is not a one-off exercise. It is dynamically evolving every day, needing constant nurture from everyone in the team. Integrating quality and compliance into your product early on will not only result in a better product that can be put on market faster but also attract more business and investment opportunities.
Yet, ever so often, we see Quality teams working in a silo from others having to constantly chase other teams via emails, meetings or needing them to log in occasionally to their system. This can get lonely.
Even the language the Quality and Development teams speak are different – developers have epics and tasks and sprints and plugins. And they definitely do not want to be slowed down by the Quality team.
Do not leave your Compliance team behind – invite them over to Jira and Confluence. They will learn to love it!
There are plenty of Risk and Quality Management apps that integrate with those designed for product development on Jira and Confluence .
If they ask you any of the following questions and you do not know the answer, send them to us – we are happy to help you.
Typical questions include:
- Is this software tool validated?
- How do the electronic signatures work?
- How to manage document lifecycle
- Can I define automated workflows?
- Is there an audit trail?
- What about permissions and security?
- Can I apply our Risk Management process using this tool?
- Can I use different Risk Management methods?
- How is it all backed up?
Check out the SoftComply Solutions on Risk Management & Document/Quality Management on Jira and Confluence for your Quality team:
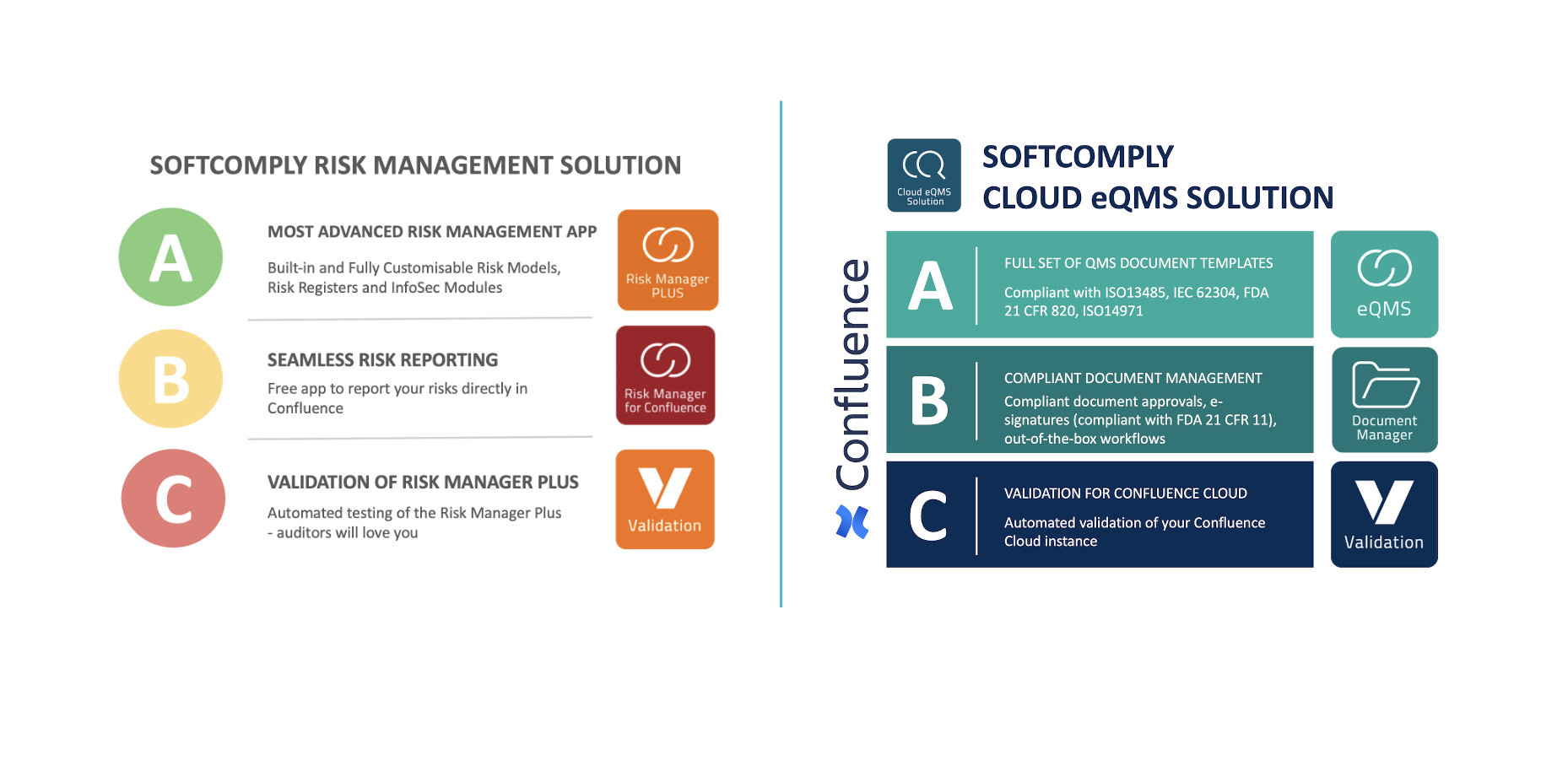
To learn more about SoftComply Solutions for your Quality and Compliance teams, please book a Live Demo call focusing on Document and Quality Management on Confluence Cloud or on Risk Management on Atlassian Cloud.
Talk soon!





