TL;DR
- Introducing the NEW & ADVANCED Document Management app on Confluence Cloud: the SoftComply Document Manager.
- BOOK A LIVE DEMO to learn more.
INTRODUCTION
Choosing the appropriate document management system for your company is a critical task. You will commit the company and employees to a specific software tools for years to come.
The choice of the correct platform depends on many factors, such as user requirements, size of the company and requirements for compliance (e.g. 21 CFR 11, GDPR, HIPAA, etc.).
After years of helping customers of different sizes and at different stages of their QMS development, we believe that Confluence is a great platform, especially if the development team is already using other Atlassian tools like Jira. Developers can have direct and seamless inputs into the records, thus reducing time and avoiding working in silos.
CONFLUENCE
Atlassian Confluence Cloud is a wiki-style document management system with integrated document editor. Despite its high customizability, the out-of-the-box version is a blank canvas where admins and users have to configure the system to suit their specific needs. This often requires the installation and configuration of Apps from the Atlassian Marketplace, a service where Confluence users can purchase add-ons for Confluence for a myriad of different purposes.
The current state-of-the-art setup of Confluence for the QMS purposes includes several Apps for workflows, electronic signatures, improved reporting, validation, change management and more creating what is often called app fatigue.
BENEFITS OF THE SOFTCOMPLY DOCUMENT MANAGER
After setting up hundreds of QMSs on Confluence for our customers, and dedicating almost two years to development, we are proud to announce that the SoftComply Document Manager is now available on the Atlassian Marketplace for Confluence Cloud.
This App includes most of the features required for an efficient and compliant Document Management system, while still using Confluence as the repository for Electronic Records.
FULLY INTEGRATED YET INDEPENDENT
The best of both worlds: central hub for your documents with the full power of Confluence. All of your content remains in Confluence but is managed through the Document Manager’s simple interface. All Confluence features and Confluence apps can be used together with the Document Manager, including Jira reporting.
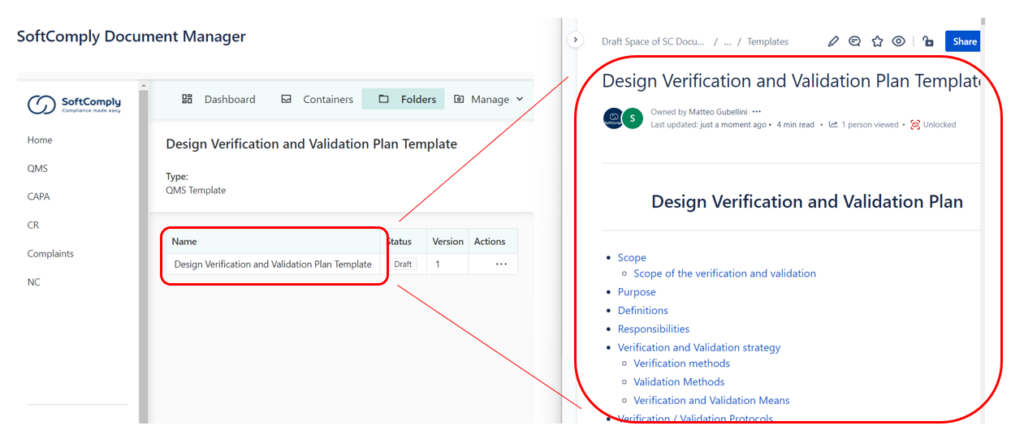
FULL DOCUMENT LIFECYCLE
Everything you need for the management of documents and records: workflows, approvals, rejections, tasks, 2-factors electronic signatures, independent versioning, due dates, release, obsoletion, advanced document access control, audit trails and more.
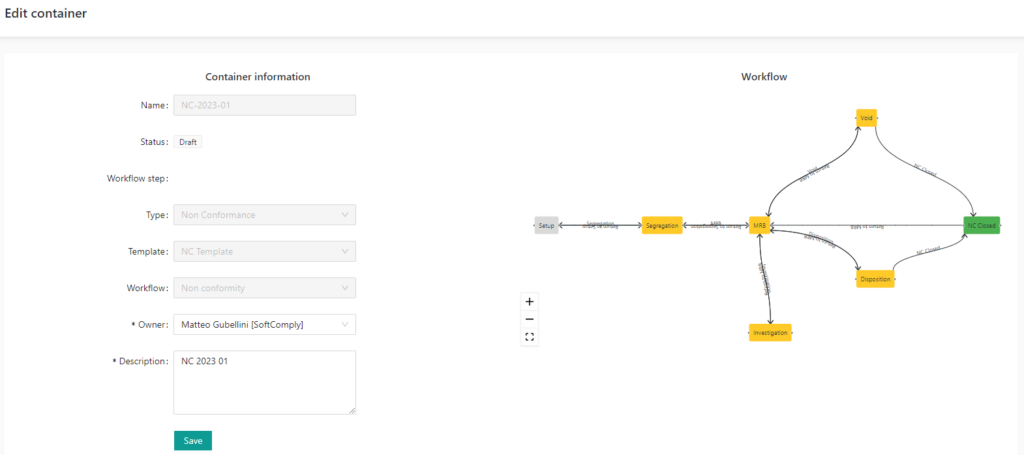
COMPLIANCE
The system is fully FDA 21 CFR 11 compliant. This includes compliant electronic signatures, electronic records protection, encryption, backups, audit trails and more.
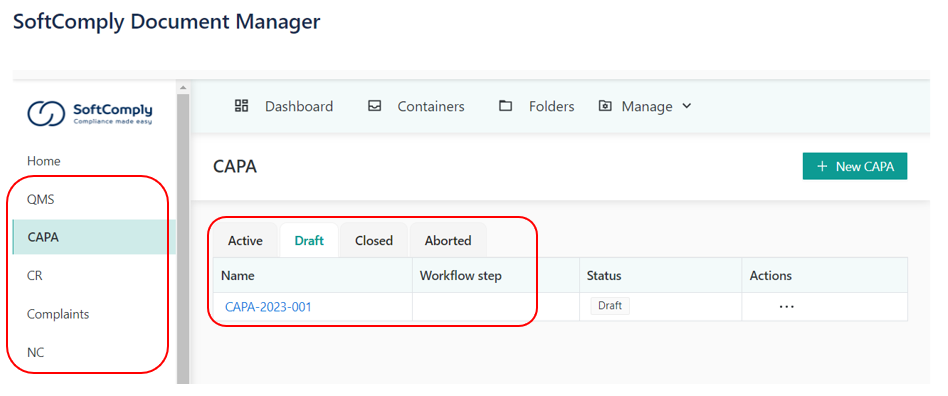
DEDICATED MODULES FOR RECORDS MANAGEMENT
The SoftComply Document Manager includes specific modules for CAPA, Non-Conformities, Change Requests and Complaints management. Pre-configured forms include all the necessary fields to manage these records. Users can also create their own forms.
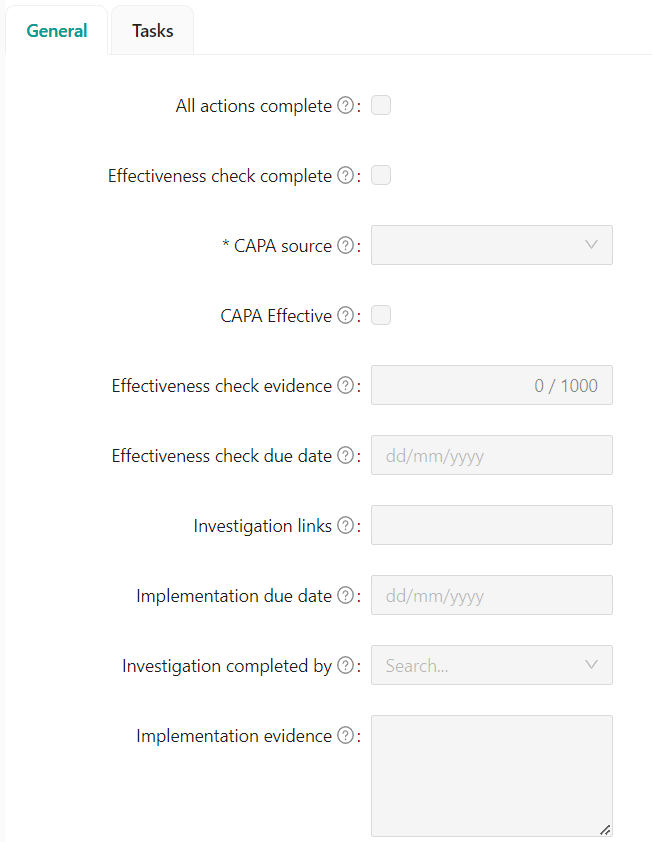
FORMS
You can now build custom Forms to capture your records instead of creating separate pages in Confluence. Forms can be used to capture the evidence for completing your CAPAs, Change Requests, and more.
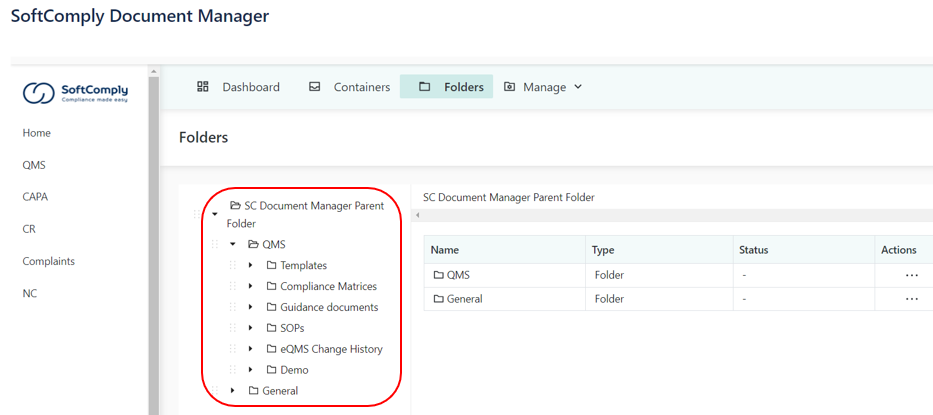
FAMILIAR DOCUMENT NAVIGATION
The content is structured using the familiar Folders view used in laptops.
MOST COMPETITIVE PRICE
The SoftComply Document Manager costs a fraction of any standalone solution while providing you similar user experience on Confluence. Pricing starts from $50 per month for up to 10 users.
HOW DOES IT WORK?
What is included out-of-the-box
- A Document Management system seamlessly integrated to Confluence Cloud;
- Electronic signatures compliant to FDA 21 CFR 11;
- 6 standard Container Templates, unlimited Custom Templates;
- Ability to configure Container Templates as Forms;
- 6 standard workflows with custom workflows developed for you upon request;
- Unlimited custom fields;
- Standard reporting for CAPA, NC, Complaints, Change Requests.
- A dedicated repository for QMS documents.
Extended Services
- SoftComply can configure your SoftComply Document Manager to include the compliant QMS documentation:
- Quality Manual and Policy;
- 25 Procedures
- 75 Templates
- Compliance matrices for 21 CFR 820, 21 CFR 11, ISO 13485, ISO 14971, IEC 62304 (all latest versions).
- SoftComply can develop custom workflows upon request to automate your existing processes and practices.
- SoftComply can provide an onboarding training (and proof of attendance) to you and your team.
HOW TO LEARN MORE
🎒 Online Live Demos of the SoftComply Document Manager every Thursday at 4pm CET / 10am EST. ✍️ Register here!
🤙 Book a LIVE DEMO with the Document Manager team!





