Confluence Cloud is constantly changing and not all the changes that are implemented by Atlassian are gated, i.e. they can’t be verified over the 2 weeks of Atlassian Release Tracks.
SoftComply Validation app for Confluence Cloud is developed to monitor changes in and ensure the data integrity of your Confluence instance. The Validation app for Confluence Cloud runs automated integrity checks of your Confluence instance once every 7 days. As a result of each run, you will have the validation protocol and validation results with documented evidence. Test Results describe each test that was run recording the documented evidence (the screenshots) highlighting the result (pass/fail) of the test.
SoftComply Validation app was published in the end of 2022 and it has detected numerous changes in Confluence Cloud over the last half year, the most significant ones regarding performance and layout were the following.
Performance-Related Changes in Confluence Cloud
On June 13th, the Validation app for Confluence Cloud detected two performance related changes in Confluence Cloud that caused a series of incidents for Confluence users. These incidents significantly impacted the performance and availability of the platform leading to issues related to user management (Bitbucket issues), latency (Forge time-out issues) and the validation process of Confluence Cloud.
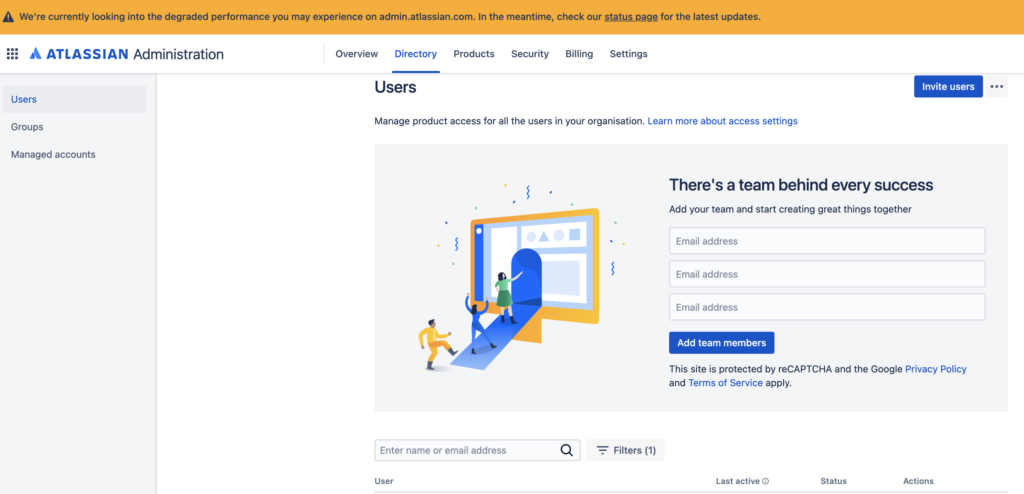
Due to issues with Bitbucket, new Confluence users could not be added on June 14:
Latency caused by Forge time-out issues affected countless number of users, especially in Confluence functionalities that tend to take time e.g. space import:
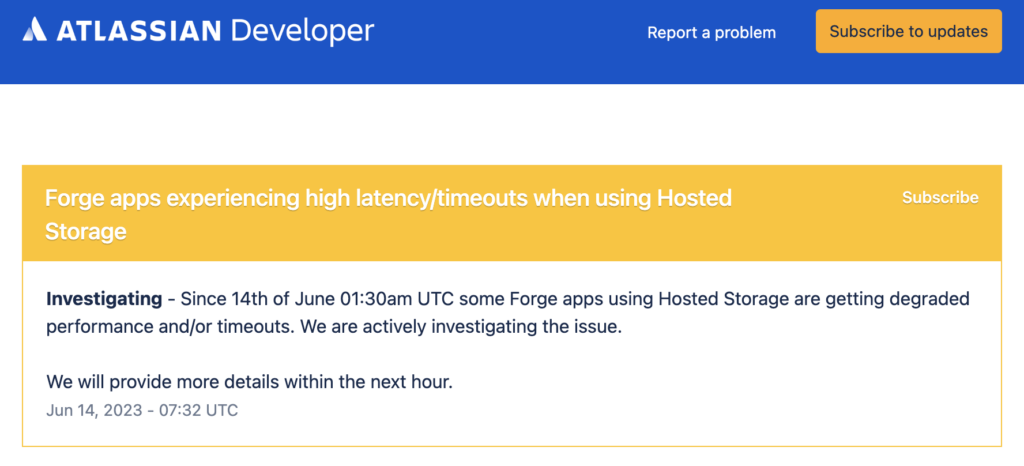
SoftComply raised these issues immediately to Atlassian who solved them in a few days time.
UI-Related Changes in Confluence Cloud
Layout and UI changes are the most frequent types of changes in Confluence Cloud. Most of them are small enough to not disrupt everyday work of Confluence users.
There are some UI changes that are a little bit more impactful. A few examples of these UI changes that the Validation app for Confluence Cloud detected are described below.
Changes in the “Create a new space” Wizard
Back in February of 2023 (on Feb 15, 2023), the Validation app for Confluence detected that the layout of the “Create a new space” wizard page had changed significantly.
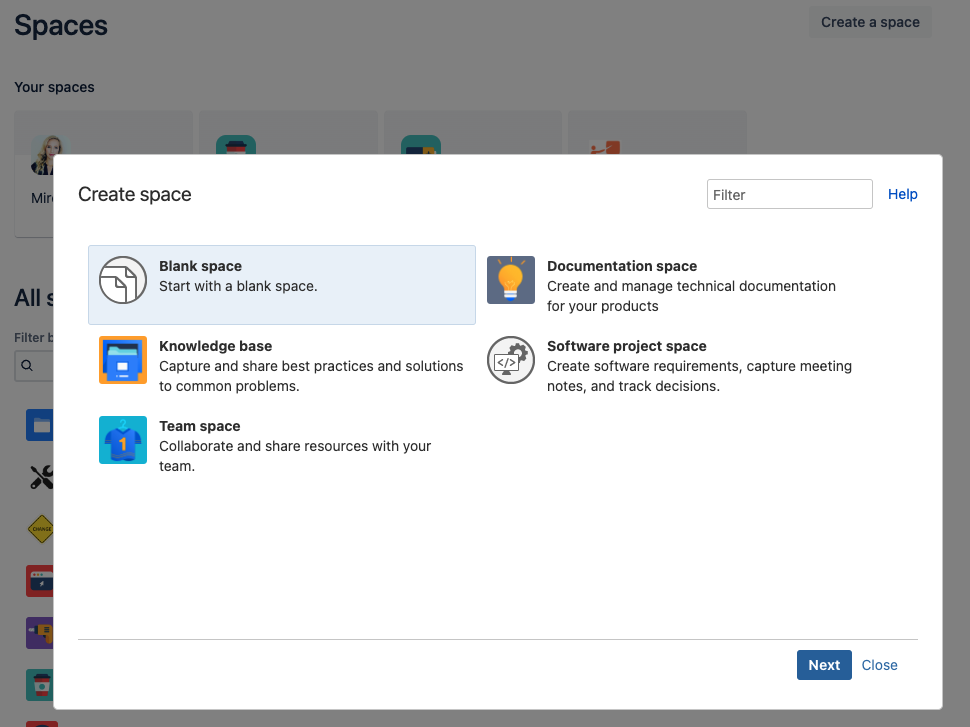
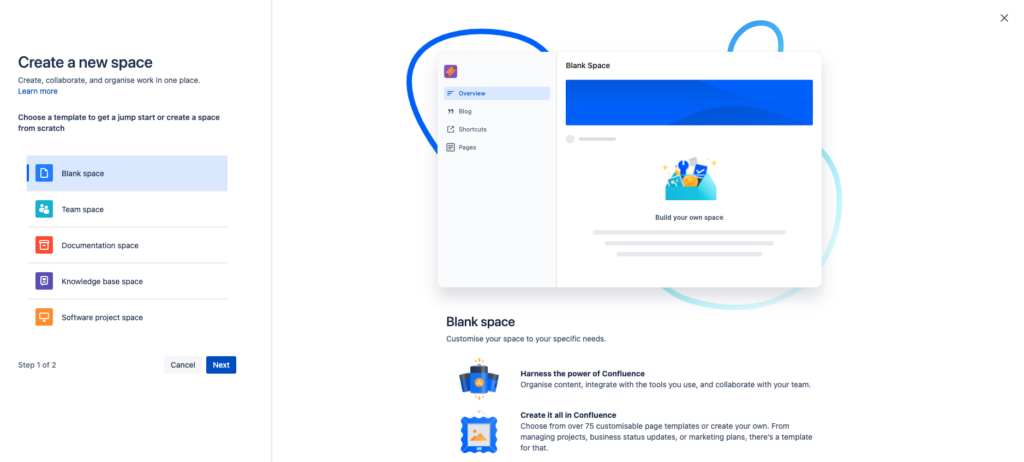
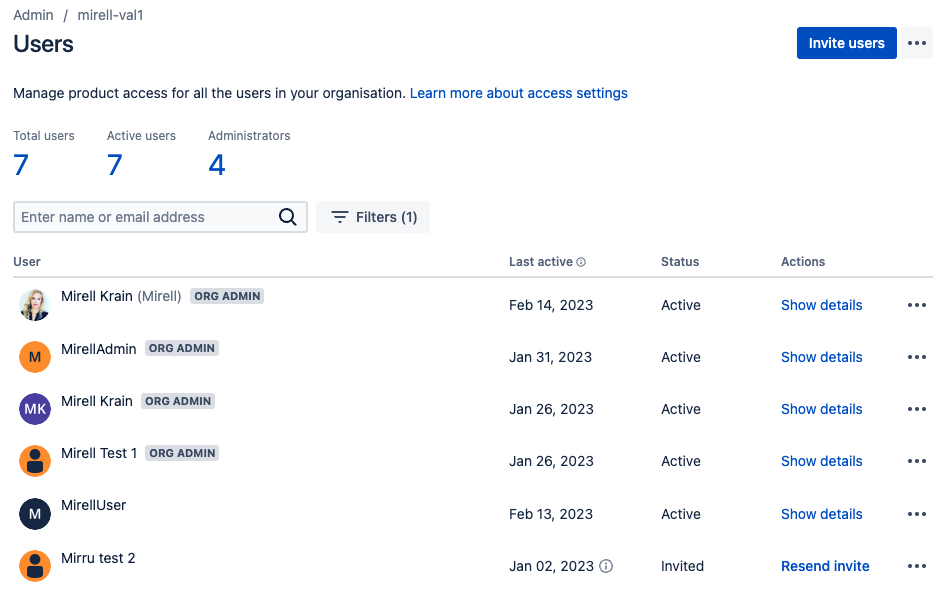
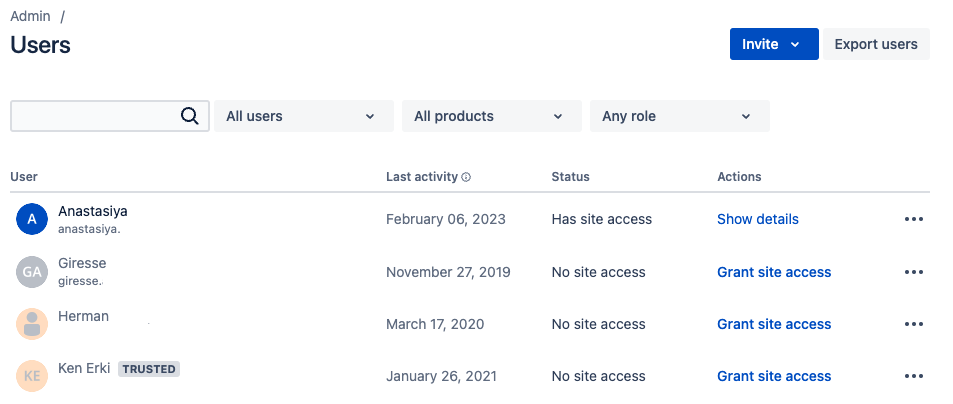
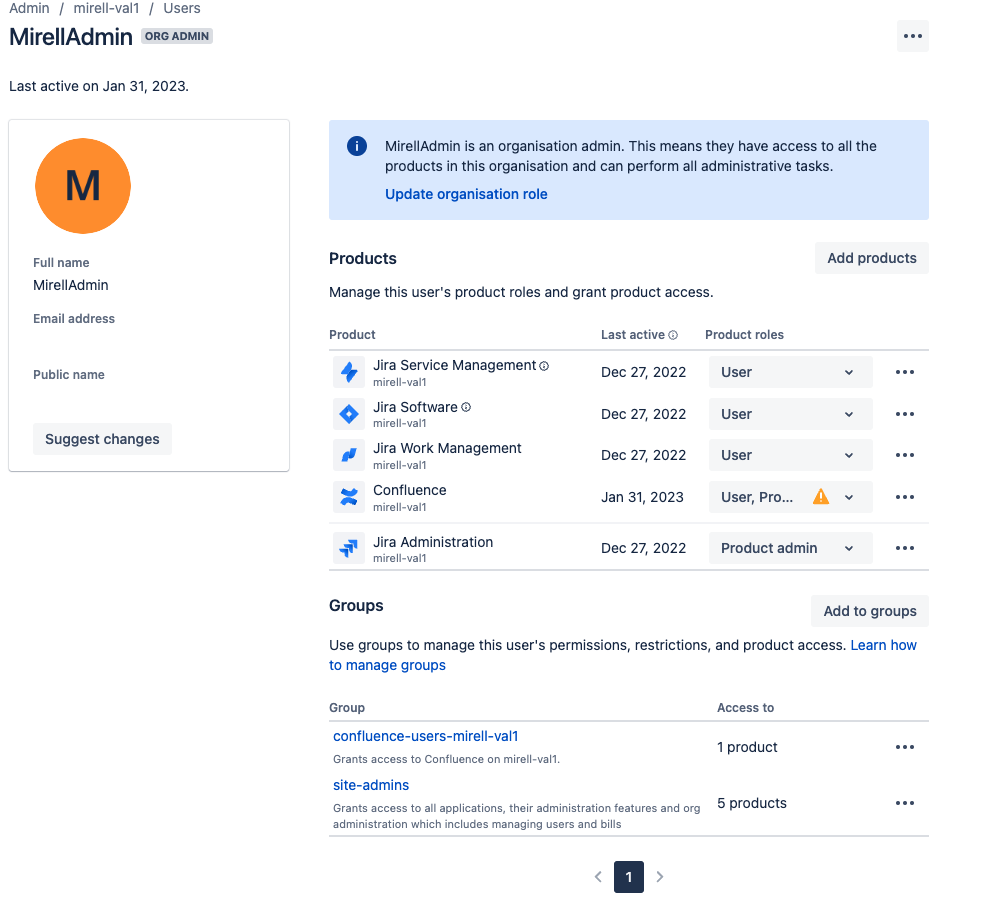
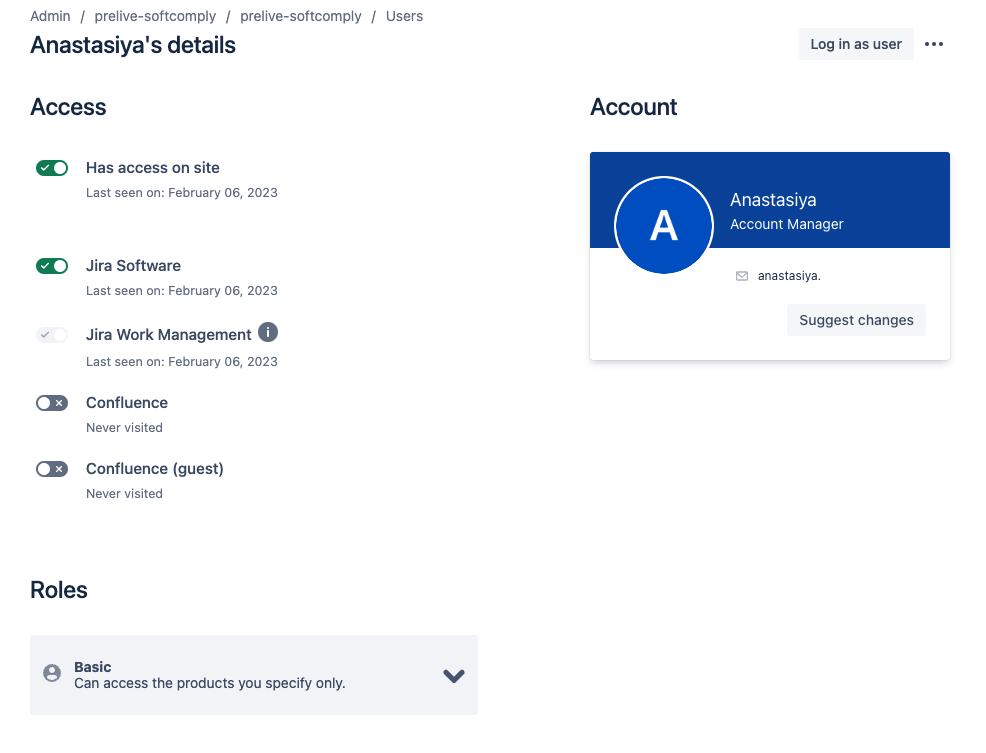
Changes on the “User Management” Pages
On the same day, Feb 15, 2023, the layout of the user management pages in Confluence Cloud changed. Both the list view of the organisation’s Confluence users as well as an individual user view changed. This change affected the admin users the most, especially for suspending users from their Confluence instance.
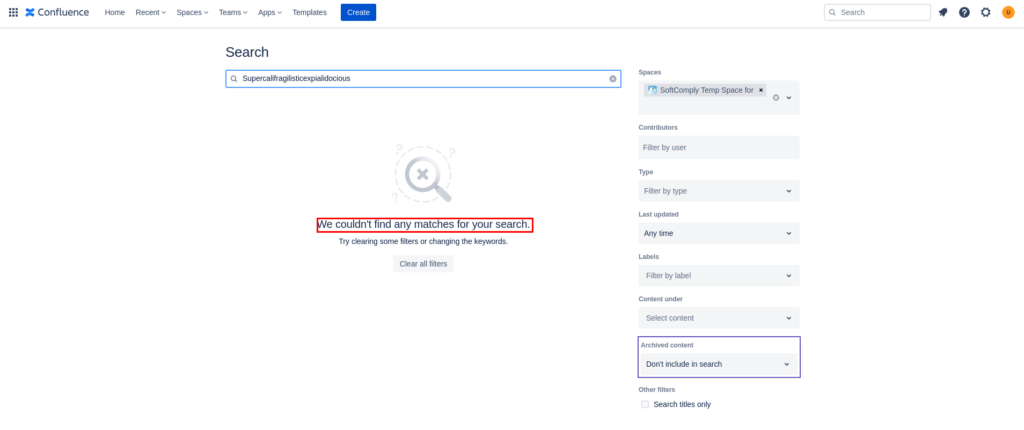
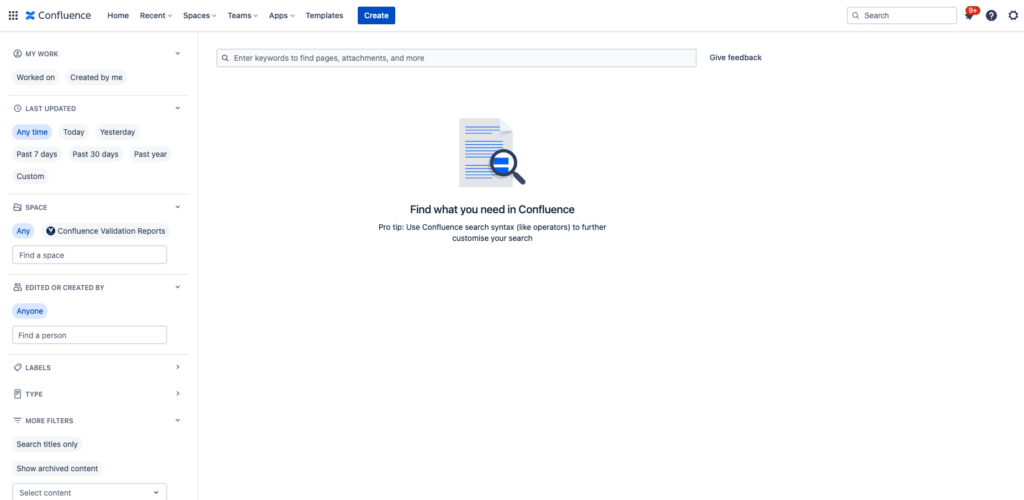
Changes on the “Advanced Search” Page
On April 13, 2023, the Validation app for Confluence Cloud detected major UI changes on the Advanced Search page.
Learn More about the Validation App
To learn more about the Validation app, feel free to book a demo call with SoftComply Validation experts:






