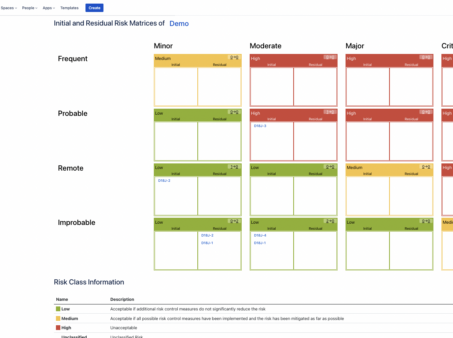
How to Report Risks in Confluence
Introduction Managing your product risks in Jira is great – you can use the flexibility of Jira…

How to Manage Requirements in Confluence and Jira
Introduction to Requirements Management in Jira and Confluence Requirements management in the regulated domains such as…
Risk Management Guide for a Digital Health or a Medical Device Company on Jira Cloud
Why Risk Management In the fast-paced world of medical device development, safety and quality are of…

How to Build a Risk Analysis in Jira
How to build a risk analysis in Jira with the SoftComply Risk Manager app
SoftComply
What is a Risk Management File?
ISO 14971:2019 defines the Risk Management file as a “set of records and other documents that…
SoftComply
What is Probability of Failure of Medical Device Software?
One of the more controversial requirements of IEC 62304 is the probability of failure of medical…