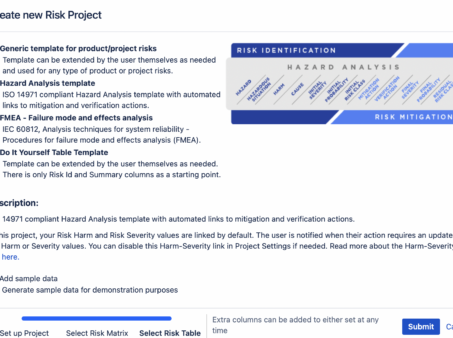
Risk Management in Jira: Streamlining the Process with Powerful Tools
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a…
How Cibiltech became FDA 21 CFR 11 Compliant on Atlassian Confluence
Customer Story on the MediCompli Solution at Cibiltech NOTE: Cibiltech has now successfully migrated from Atlassian…