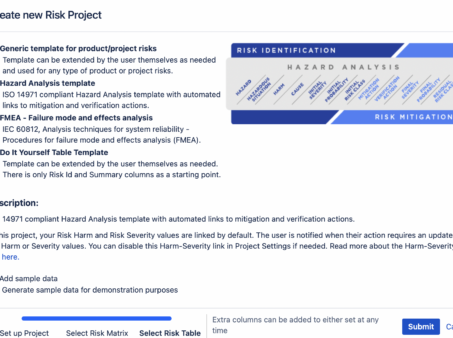
Risk Management in Jira: Streamlining the Process with Powerful Tools
Risk Management in Jira In today’s fast-paced and ever-evolving business landscape, effective risk management plays a…
SoftComply
How to Add Page Numbers to the PDF Exports of Quality Records in Confluence
Paper-based Quality Management Systems are now (almost) a thing of the past (it’s sooo 20th century…).…
SoftComply
How to Use Confluence Pages as Templates
Summary SoftComply has released a new Confluence Server macro within the SoftComply eQMS app that allows…
SoftComply
What is a Risk Management File?
ISO 14971:2019 defines the Risk Management file as a “set of records and other documents that…
SoftComply
How I came to hate Excel & decided to develop an automated Risk Management tool for JIRA
Part II By Matteo Gubellini, VP of Regulatory Affairs at SoftComply* So you are looking for…
SoftComply
How I came to hate Excel & decided to develop an automated Risk Management tool for JIRA
Part I By Matteo Gubellini, VP of Regulatory Affairs of SoftComply* “Ok, let’s follow a few…