Pricing starts from
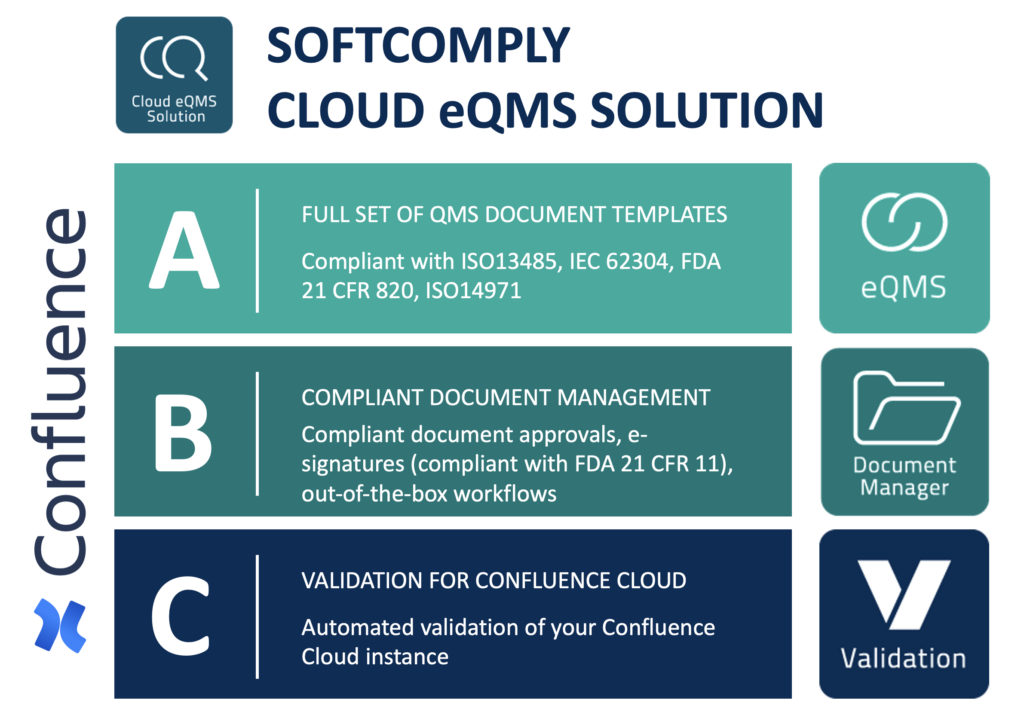
A - QMS Content
$
2400
one-off (if needed)
Compliant QMS Content
Get FDA and MDR compliant QMS content from SoftComply eQMS app.
Over 25 SOPs and 75 Technical Document Templates - pre-filled with embedded guidance.
B - SoftComply Document Manager
$
47
per month
App for Workflows and E-signatures
Confluence app for automating Document Approval Workflows with FDA 21 CFR 11 Compliant E-signatures: SoftComply Document Manager on Confluence Cloud.
C - Validation for Confluence Cloud
$
50
per month
Regular Integrity Checks of Confluence Cloud
Confluence app to run automated Validation tests of your Confluence Cloud instance 1 x a week generating full Validation Documentation pack as a result of each test run.
Configuration
$
1000
one-off
Confluence Configuration Service & Onboarding Training
SoftComply team configures your eQMS Content as needed, and provides an On-Boarding Training of the Solution to your organisation.
Comparison
FEATURE
Best Fit for SaMD (Software as a Medical Device)
Possibility to Trial
SME Price Range
Bi-Directional Integration with Jira
MasterControl




SoftComply Cloud eQMS




Greenlight Guru




Qualio




Cloud eQMS Solution Architecture
Book a Demo Call